Influence of mycotoxins and a mycotoxin adsorbing agent on the oral bioavailability of commonly used antibiotics in pigs
- PMID: 22606377
- PMCID: PMC3347004
- DOI: 10.3390/toxins4040281
Influence of mycotoxins and a mycotoxin adsorbing agent on the oral bioavailability of commonly used antibiotics in pigs
Abstract
It is recognized that mycotoxins can cause a variety of adverse health effects in animals, including altered gastrointestinal barrier function. It is the aim of the present study to determine whether mycotoxin-contaminated diets can alter the oral bioavailability of the antibiotics doxycycline and paromomycin in pigs, and whether a mycotoxin adsorbing agent included into diets interacts with those antibiotics. Experiments were conducted with pigs utilizing diets that contained blank feed, mycotoxin-contaminated feed (T-2 toxin or deoxynivalenol), mycotoxin-contaminated feed supplemented with a glucomannan mycotoxin binder, or blank feed supplemented with mycotoxin binder. Diets with T-2 toxin and binder or deoxynivalenol and binder induced increased plasma concentrations of doxycycline administered as single bolus in pigs compared to diets containing blank feed. These results suggest that complex interactions may occur between mycotoxins, mycotoxin binders, and antibiotics which could alter antibiotic bioavailability. This could have consequences for animal toxicity, withdrawal time for oral antibiotics, or public health.
Keywords: antibiotics; interaction; mycotoxin binder; mycotoxins; pigs; safety testing.
Figures
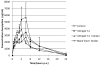

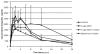

References
-
- Monbaliu S., van Poucke C., Detavernier C., Dumoulin F., van de Velde M., Schoeters E., van Dyck S., Averkieva O., van Peteghem C., de Saeger S. Occurrence of mycotoxins in feed as analyzed by a multi-mycotoxin LC-MS/MS method. J. Agric. Food Chem. 2010;58:66–71. - PubMed
-
- Lambert D., Padfield P.J., McLaughlin J., Cannell S., O’Neill C.A. Ochratoxin A displaces claudins from detergent resistant membrane microdomains. Biochem. Biophys. Res. Commun. 2007;358:632–636. - PubMed
-
- Mahfoud R., Maresca M., Garmy N., Fantini J. The mycotoxin patulin alters the barrier function of the intestinal epithelium: Mechanism of action of the toxin and protective effects of glutathione. Toxicol. Appl. Pharmacol. 2002;181:209–218. - PubMed
-
- Maresca M., Mahfoud R., Pfohl-Leszkowicz A., Fantini J. The mycotoxin ochratoxin A alters intestinal barrier and absorption functions but has no effect on chloride secretion. Toxicol. Appl. Pharmacol. 2001;176:54–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

