Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis
- PMID: 22607134
- PMCID: PMC3422642
- DOI: 10.1056/NEJMoa1113354
Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis
Abstract
Background: A combination of prednisone, azathioprine, and N-acetylcysteine (NAC) has been widely used as a treatment for idiopathic pulmonary fibrosis. The safety and efficacy of this three-drug regimen is unknown.
Methods: In this randomized, double-blind, placebo-controlled trial, we assigned patients with idiopathic pulmonary fibrosis who had mild-to-moderate lung-function impairment to one of three groups -- receiving a combination of prednisone, azathioprine, and NAC (combination therapy), NAC alone, or placebo -- in a 1:1:1 ratio. The primary outcome was the change in longitudinal measurements of forced vital capacity during a 60-week treatment period.
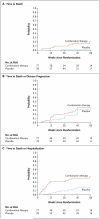
Results: When approximately 50% of data had been collected (with 77 patients in the combination-therapy group and 78 in the placebo group), a planned interim analysis revealed that patients in the combination-therapy group, as compared with the placebo group, had an increased rate of death (8 vs. 1, P=0.01) and hospitalization (23 vs. 7, P<0.001). These observations, coupled with no evidence of physiological or clinical benefit for combination therapy, prompted the independent data and safety monitoring board to recommend termination of the combination-therapy group at a mean follow-up of 32 weeks. Data from the ongoing comparison of the NAC-only group and the placebo group are not reported here.
Conclusions: Increased risks of death and hospitalization were observed in patients with idiopathic pulmonary fibrosis who were treated with a combination of prednisone, azathioprine, and NAC, as compared with placebo. These findings provide evidence against the use of this combination in such patients. (Funded by the National Heart, Lung, and Blood Institute and the Cowlin Family Fund; ClinicalTrials.gov number, NCT00650091.).
Figures


Comment in
-
Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis.N Engl J Med. 2012 Aug 30;367(9):869; author reply 870-1. doi: 10.1056/NEJMc1207471. N Engl J Med. 2012. PMID: 22931324 No abstract available.
-
Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis.N Engl J Med. 2012 Aug 30;367(9):870; author reply 870-1. doi: 10.1056/NEJMc1207471. N Engl J Med. 2012. PMID: 22931325 No abstract available.
-
Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis.N Engl J Med. 2012 Aug 30;367(9):870; author reply 870-1. doi: 10.1056/NEJMc1207471. N Engl J Med. 2012. PMID: 22931326 No abstract available.
References
-
- American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement. Am J Respir Crit Care Med. 2000;161:646–64. - PubMed
-
- Peikert T, Daniels C, Beebe T, Meyer K, Ryu JH. Assessment of current practice in the diagnosis and therapy of idiopathic pulmonary fibrosis. Respir Med. 2008;102:1342–8. - PubMed
-
- Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. - PubMed
-
- Hunninghake GW. Antioxidant therapy for idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2285–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K24 HL111316/HL/NHLBI NIH HHS/United States
- U10 HL080413/HL/NHLBI NIH HHS/United States
- U10 HL080513/HL/NHLBI NIH HHS/United States
- U10 HL080543/HL/NHLBI NIH HHS/United States
- U10HL080510/HL/NHLBI NIH HHS/United States
- U10 HL080685/HL/NHLBI NIH HHS/United States
- U10HL080413/HL/NHLBI NIH HHS/United States
- U10HL080383/HL/NHLBI NIH HHS/United States
- U10HL080371/HL/NHLBI NIH HHS/United States
- U10 HL080274/HL/NHLBI NIH HHS/United States
- U10HL080513/HL/NHLBI NIH HHS/United States
- U10HL080685/HL/NHLBI NIH HHS/United States
- R01 HL105479/HL/NHLBI NIH HHS/United States
- U10 HL080371/HL/NHLBI NIH HHS/United States
- U10HL080411/HL/NHLBI NIH HHS/United States
- U10 HL080383/HL/NHLBI NIH HHS/United States
- R01 HL089249/HL/NHLBI NIH HHS/United States
- U10HL080370/HL/NHLBI NIH HHS/United States
- U10 HL080370/HL/NHLBI NIH HHS/United States
- U10HL080571/HL/NHLBI NIH HHS/United States
- U10 HL080411/HL/NHLBI NIH HHS/United States
- U10HL080509/HL/NHLBI NIH HHS/United States
- U10HL080274/HL/NHLBI NIH HHS/United States
- U10 HL080509/HL/NHLBI NIH HHS/United States
- U10 HL080571/HL/NHLBI NIH HHS/United States
- U10 HL080510/HL/NHLBI NIH HHS/United States
- U10HL080543/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
