Overexpression of an exotic thermotolerant β-glucosidase in trichoderma reesei and its significant increase in cellulolytic activity and saccharification of barley straw
- PMID: 22607229
- PMCID: PMC3434039
- DOI: 10.1186/1475-2859-11-63
Overexpression of an exotic thermotolerant β-glucosidase in trichoderma reesei and its significant increase in cellulolytic activity and saccharification of barley straw
Abstract
Background: Trichoderma reesei is a widely used industrial strain for cellulase production, but its low yield of β-glucosidase has prevented its industrial value. In the hydrolysis process of cellulolytic residues by T. reesei, a disaccharide known as cellobiose is produced and accumulates, which inhibits further cellulases production. This problem can be solved by adding β-glucosidase, which hydrolyzes cellobiose to glucose for fermentation. It is, therefore, of high vvalue to construct T. reesei strains which can produce sufficient β-glucosidase and other hydrolytic enzymes, especially when those enzymes are capable of tolerating extreme conditions such as high temperature and acidic or alkali pH.
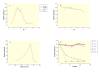
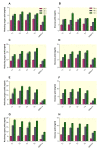
Results: We successfully engineered a thermostable β-glucosidase gene from the fungus Periconia sp. into the genome of T. reesei QM9414 strain. The engineered T. reesei strain showed about 10.5-fold (23.9 IU/mg) higher β-glucosidase activity compared to the parent strain (2.2 IU/mg) after 24 h of incubation. The transformants also showed very high total cellulase activity (about 39.0 FPU/mg) at 24 h of incubation whereas the parent strain almost did not show any total cellulase activity at 24 h of incubation. The recombinant β-glucosidase showed to be thermotolerant and remains fully active after two-hour incubation at temperatures as high as 60°C. Additionally, it showed to be active at a wide pH range and maintains about 88% of its maximal activity after four-hour incubation at 25°C in a pH range from 3.0 to 9.0. Enzymatic hydrolysis assay using untreated, NaOH, or Organosolv pretreated barley straw as well as microcrystalline cellulose showed that the transformed T. reesei strains released more reducing sugars compared to the parental strain.
Conclusions: The recombinant T. reesei overexpressing Periconia sp. β-glucosidase in this study showed higher β-glucosidase and total cellulase activities within a shorter incubation time (24 h) as well as higher hydrolysis activity using biomass residues. These features suggest that the transformants can be used for β-glucosidase production as well as improving the biomass conversion using cellulases.
Figures




Similar articles
-
Revisiting overexpression of a heterologous β-glucosidase in Trichoderma reesei: fusion expression of the Neosartorya fischeri Bgl3A to cbh1 enhances the overall as well as individual cellulase activities.Microb Cell Fact. 2016 Jul 11;15(1):122. doi: 10.1186/s12934-016-0520-9. Microb Cell Fact. 2016. PMID: 27400964 Free PMC article.
-
A β-glucosidase hyper-production Trichoderma reesei mutant reveals a potential role of cel3D in cellulase production.Microb Cell Fact. 2016 Sep 1;15(1):151. doi: 10.1186/s12934-016-0550-3. Microb Cell Fact. 2016. PMID: 27585813 Free PMC article.
-
Saccharification of rice straw by cellulase from a local Trichoderma harzianum SNRS3 for biobutanol production.BMC Biotechnol. 2014 Dec 12;14:103. doi: 10.1186/s12896-014-0103-y. BMC Biotechnol. 2014. PMID: 25496491 Free PMC article.
-
[Mechanisms and regulation of enzymatic hydrolysis of cellulose in filamentous fungi: classical cases and new models].Rev Iberoam Micol. 2015 Jan-Mar;32(1):1-12. doi: 10.1016/j.riam.2013.10.009. Epub 2014 Mar 7. Rev Iberoam Micol. 2015. PMID: 24607657 Review. Spanish.
-
Deciphering the molecular mechanisms behind cellulase production in Trichoderma reesei, the hyper-cellulolytic filamentous fungus.Biosci Biotechnol Biochem. 2016 Sep;80(9):1712-29. doi: 10.1080/09168451.2016.1171701. Epub 2016 Apr 14. Biosci Biotechnol Biochem. 2016. PMID: 27075508 Review.
Cited by
-
Microbial enzymes: tools for biotechnological processes.Biomolecules. 2014 Jan 16;4(1):117-39. doi: 10.3390/biom4010117. Biomolecules. 2014. PMID: 24970208 Free PMC article. Review.
-
Genetic engineering of Trichoderma reesei cellulases and their production.Microb Biotechnol. 2017 Nov;10(6):1485-1499. doi: 10.1111/1751-7915.12726. Epub 2017 May 29. Microb Biotechnol. 2017. PMID: 28557371 Free PMC article. Review.
-
Improved Production of Majority Cellulases in Trichoderma reesei by Integration of cbh1 Gene From Chaetomium thermophilum.Front Microbiol. 2020 Jul 14;11:1633. doi: 10.3389/fmicb.2020.01633. eCollection 2020. Front Microbiol. 2020. PMID: 32765463 Free PMC article.
-
Characterization of Aspergillus aculeatus β-glucosidase 1 accelerating cellulose hydrolysis with Trichoderma cellulase system.AMB Express. 2015 Jan 24;5(1):3. doi: 10.1186/s13568-014-0090-3. eCollection 2015 Dec. AMB Express. 2015. PMID: 25642400 Free PMC article.
-
Crystal structure and biochemical characterization of the recombinant ThBgl, a GH1 β-glucosidase overexpressed in Trichoderma harzianum under biomass degradation conditions.Biotechnol Biofuels. 2016 Mar 22;9:71. doi: 10.1186/s13068-016-0487-0. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27006690 Free PMC article.
References
-
- Seidl V, Seiboth B. Trichoderma reesei: genetic approaches to improving strain efficiency. Biofuels. 2010;1(2):343–354. doi: 10.4155/bfs.10.1. - DOI
-
- Messner R, Kubicek CP. Evidence for a single, specific β-glucosidase in cell walls from Trichoderma reesei QM 9414. Enzyme Microb Technol. 1990;12:685–690. doi: 10.1016/0141-0229(90)90008-E. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

