Increasing levels of wild-type CREB up-regulates several activity-regulated inhibitor of death (AID) genes and promotes neuronal survival
- PMID: 22607375
- PMCID: PMC3407521
- DOI: 10.1186/1471-2202-13-48
Increasing levels of wild-type CREB up-regulates several activity-regulated inhibitor of death (AID) genes and promotes neuronal survival
Abstract
Background: CREB (cAMP-response element binding protein) is the prototypical signal-regulated transcription factor. In neurons, it is the target of the synaptic activity-induced nuclear calcium-calcium/calmodulin dependent protein kinase (CaMK) IV signaling pathway that controls the expression of genes important for acquired neuroprotection as well as other long-lasting adaptive processes in the nervous system. The function of CREB as a transcriptional activator is controlled by its phosphorylation on serine 133, which can be catalyzed by CaMKIV and leads to the recruitment of the co-activator, CREB binding protein (CBP). Activation of CBP function by nuclear calcium-CaMKIV signaling is a second regulatory step required for CREB/CBP-mediated transcription.
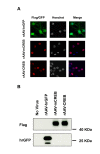
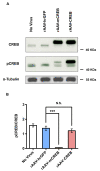
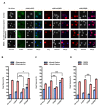
Results: Here we used recombinant adeno-associated virus (rAAV) to increase the levels of wild type CREB or to overexpress a mutant version of CREB (mCREB) containing a serine to alanine mutation at position amino acid 133 in mouse hippocampal neurons. Increasing the levels of CREB was sufficient to boost neuroprotective activity even under basal conditions (i.e., in the absence of stimulation of synaptic activity). In contrast, overexpression of mCREB increased cell death. The ratio of phospho(serine 133)CREB to CREB immunoreactivity in unstimulated hippocampal neurons was similar for endogenous CREB and overexpressed wild type CREB and, as expected, dramatically reduced for overexpressed mCREB. A gene expression analysis revealed that increased expression of CREB but not that of mCREB in hippocampal neurons led to elevated expression levels of bdnf as well as that of several members of a previously characterized set of Activity-regulated Inhibitor of Death (AID) genes, which include atf3, btg2, gadd45β, and gadd45γ.
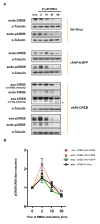
Conclusions: Our findings indicate that the expression levels of wild type CREB are a critical determinant of the ability of hippocampal neurons to survive harmful conditions. Increasing the levels of wild type CREB can, even without inducing synaptic activity, increase pro-survival gene expression and strengthen the neurons' neuroprotective shield. The observed degradation of CREB protein following NMDA treatment of hippocampal neurons suggests that the known CREB shut-off associated with extrasynaptic NMDA receptor-induced excitotoxicity is followed by CREB proteolysis.
Figures
Similar articles
-
A signaling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage.J Neurosci. 2011 Mar 30;31(13):4978-90. doi: 10.1523/JNEUROSCI.2672-10.2011. J Neurosci. 2011. PMID: 21451036 Free PMC article.
-
Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo.J Neurosci. 2001 Dec 1;21(23):9204-13. doi: 10.1523/JNEUROSCI.21-23-09204.2001. J Neurosci. 2001. PMID: 11717354 Free PMC article.
-
Nuclear calcium signaling controls methyl-CpG-binding protein 2 (MeCP2) phosphorylation on serine 421 following synaptic activity.J Biol Chem. 2012 Sep 7;287(37):30967-74. doi: 10.1074/jbc.M112.382507. Epub 2012 Jul 20. J Biol Chem. 2012. PMID: 22822052 Free PMC article.
-
Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV.Neuron. 2002 Apr 11;34(2):235-44. doi: 10.1016/s0896-6273(02)00654-2. Neuron. 2002. PMID: 11970865
-
Calmodulin kinase IV-dependent CREB activation is required for neuroprotection via NMDA receptor-PSD95 disruption.J Neurochem. 2013 Jul;126(2):274-87. doi: 10.1111/jnc.12176. Epub 2013 Mar 3. J Neurochem. 2013. PMID: 23363435
Cited by
-
Amniotic LPS-Induced Apoptosis in the Fetal Brain Is Suppressed by Vaginal LPS Preconditioning but Is Promoted by Continuous Ischemic Reperfusion.Int J Mol Sci. 2022 Feb 4;23(3):1787. doi: 10.3390/ijms23031787. Int J Mol Sci. 2022. PMID: 35163709 Free PMC article.
-
Enriched environment remedies cognitive dysfunctions and synaptic plasticity through NMDAR-Ca2+-Activin A circuit in chronic cerebral hypoperfusion rats.Aging (Albany NY). 2021 Aug 30;13(16):20748-20761. doi: 10.18632/aging.203462. Epub 2021 Aug 30. Aging (Albany NY). 2021. PMID: 34462377 Free PMC article.
-
Coumarin-chalcone hybrid LM-021 and indole derivative NC009-1 targeting inflammation and oxidative stress to protect BE(2)-M17 cells against α-synuclein toxicity.Aging (Albany NY). 2023 Aug 11;15(16):8061-8089. doi: 10.18632/aging.204954. Epub 2023 Aug 11. Aging (Albany NY). 2023. PMID: 37578928 Free PMC article.
-
MicroRNA-219 alleviates glutamate-induced neurotoxicity in cultured hippocampal neurons by targeting calmodulin-dependent protein kinase II gamma.Neural Regen Res. 2018 Jul;13(7):1216-1224. doi: 10.4103/1673-5374.235059. Neural Regen Res. 2018. PMID: 30028330 Free PMC article.
-
Optogenetic spinal stimulation promotes new axonal growth and skilled forelimb recovery in rats with sub-chronic cervical spinal cord injury.J Neural Eng. 2023 Sep 12;20(5):056005. doi: 10.1088/1741-2552/acec13. J Neural Eng. 2023. PMID: 37524080 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

