Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus
- PMID: 22607552
- PMCID: PMC3413582
- DOI: 10.1186/1742-2094-9-95
Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus
Abstract
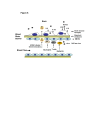
Background: Congenital human cytomegalovirus (HCMV) infections can result in CNS abnormalities in newborn babies including vision loss, mental retardation, motor deficits, seizures, and hearing loss. Brain pericytes play an essential role in the development and function of the blood-brain barrier yet their unique role in HCMV dissemination and neuropathlogy has not been reported.
Methods: Primary human brain vascular pericytes were exposed to a primary clinical isolate of HCMV designated 'SBCMV'. Infectivity was analyzed by microscopy, immunofluorescence, Western blot, and qRT-PCR. Microarrays were performed to identify proinflammatory cytokines upregulated after SBCMV exposure, and the results validated by real-time quantitative polymerase chain reaction (qPCR) methodology. In situ cytokine expression of pericytes after exposure to HCMV was examined by ELISA and in vivo evidence of HCMV infection of brain pericytes was shown by dual-labeled immunohistochemistry.
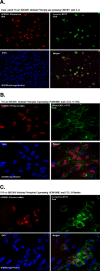
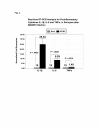
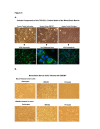
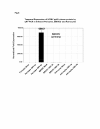
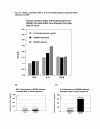
Results: HCMV-infected human brain vascular pericytes as evidenced by several markers. Using a clinical isolate of HCMV (SBCMV), microscopy of infected pericytes showed virion production and typical cytomegalic cytopathology. This finding was confirmed by the expression of major immediate early and late virion proteins and by the presence of HCMV mRNA. Brain pericytes were fully permissive for CMV lytic replication after 72 to 96 hours in culture compared to human astrocytes or human brain microvascular endothelial cells (BMVEC). However, temporal transcriptional expression of pp65 virion protein after SBCMV infection was lower than that seen with the HCMV Towne laboratory strain. Using RT-PCR and dual-labeled immunofluorescence, proinflammatory cytokines CXCL8/IL-8, CXCL11/ITAC, and CCL5/Rantes were upregulated in SBCMV-infected cells, as were tumor necrosis factor-alpha (TNF-alpha), interleukin-1 beta (IL-1beta), and interleukin-6 (IL-6). Pericytes exposed to SBCMV elicited higher levels of IL-6 compared to both mock-infected as well as heat-killed virus controls. A 6.6-fold induction of IL-6 and no induction TNF-alpha was observed in SBCMV-infected cell supernatants at 24 hours postinfection. Using archival brain tissue from a patient coinfected with HCMV and HIV, we also found evidence of HCMV infection of pericytes using dual-label immunohistochemistry, as monitored by NG2 proteoglycan staining.
Conclusion: HCMV lytic infection of primary human brain pericytes suggests that pericytes contribute to both virus dissemination in the CNS as well as neuroinflammation.
Figures



References
-
- Sessions CF, Taeusch HW. In: Viral infections of the newborn. In Diseases of the newborn. Taeusch HW, Ballard RA, Avery ME, editor. W. B. Saunders, Philadelphia; 1991. pp. 331–349.
-
- Demmler GJ. Congenital cytomegalovirus infection and disease. Adv Pediatr Infect Dis. 1996;11:135–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

