Robust spinal neuroinflammation mediates mechanical allodynia in Walker 256 induced bone cancer rats
- PMID: 22607655
- PMCID: PMC3443428
- DOI: 10.1186/1756-6606-5-16
Robust spinal neuroinflammation mediates mechanical allodynia in Walker 256 induced bone cancer rats
Abstract
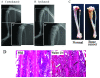
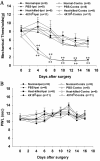
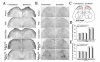
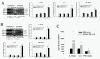
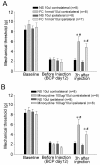
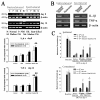
It has been reported that remarkable and sustained activation of astrocytes and/or microglia occurs in cancer induced pain (CIP), which is different from neuropathic and inflammatory pain. The present study was designed to investigate the role of spinal Toll-like receptor 4 (TLR4) induced glial neuroinflammation in cancer induced pain using a modified rat model of bone cancer. The rat model of CIP consisted of unilateral intra-tibial injection with Walker 256 mammary gland carcinoma. Nine days after Walker 256 inoculation, a robust activation of both astrocytes and microglia in bilateral spinal dorsal horn was observed together with significant bilateral mechanical allodynia. This neuroinflammation was characterized by enhanced immunostaining of both glial fibrillary acidic protein (GFAP, astrocyte marker) and OX-42 (microglia marker), and an elevated level of IL-1β, IL-6 and TNF-α mRNA. I.t. administration of fluorocitrate (an inhibitor of glial metabolism, 1 nmol) or minocycline (an inhibitor of microglia, 100 μg) has significant anti-allodynic effects on day 12 after Walker 256 inoculation. Naloxone (a nonstereoselective TLR4 signaling blocker, 60 μg, i.t.) also significantly alleviated mechanical allodynia and simultaneously blocked the increased inflammatory cytokine mRNA. The results suggested that spinal TLR4 might play an important role in the sustained glial activation that critically contributed to the robust and sustained spinal neuroinflammation in CIP. This result could potentially help clinicians and researchers to better understand the mechanism of complicated cancer pain.
Figures
Similar articles
-
Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia.Pain. 2005 Nov;118(1-2):125-36. doi: 10.1016/j.pain.2005.08.001. Epub 2005 Sep 9. Pain. 2005. PMID: 16154703
-
Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats.Spine (Phila Pa 1976). 2008 May 20;33(12):1344-51. doi: 10.1097/BRS.0b013e3181733188. Spine (Phila Pa 1976). 2008. PMID: 18496347
-
Down-Regulation of CXCL12/CXCR4 Expression Alleviates Ischemia-Reperfusion-Induced Inflammatory Pain via Inhibiting Glial TLR4 Activation in the Spinal Cord.PLoS One. 2016 Oct 19;11(10):e0163807. doi: 10.1371/journal.pone.0163807. eCollection 2016. PLoS One. 2016. PMID: 27760212 Free PMC article.
-
Targeting astrocyte signaling for chronic pain.Neurotherapeutics. 2010 Oct;7(4):482-93. doi: 10.1016/j.nurt.2010.05.016. Neurotherapeutics. 2010. PMID: 20880510 Free PMC article. Review.
-
Effects of astrocytes and microglia on neuroinflammation after spinal cord injury and related immunomodulatory strategies.Int Immunopharmacol. 2022 Jul;108:108754. doi: 10.1016/j.intimp.2022.108754. Epub 2022 Apr 6. Int Immunopharmacol. 2022. PMID: 35397392 Review.
Cited by
-
Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18.J Neurosci. 2015 May 20;35(20):7950-63. doi: 10.1523/JNEUROSCI.5250-14.2015. J Neurosci. 2015. PMID: 25995479 Free PMC article.
-
Current studies of acupuncture in cancer-induced bone pain animal models.Evid Based Complement Alternat Med. 2014;2014:191347. doi: 10.1155/2014/191347. Epub 2014 Oct 14. Evid Based Complement Alternat Med. 2014. PMID: 25383081 Free PMC article. Review.
-
The analgesic effects of triptolide in the bone cancer pain rats via inhibiting the upregulation of HDACs in spinal glial cells.J Neuroinflammation. 2017 Nov 2;14(1):213. doi: 10.1186/s12974-017-0988-1. J Neuroinflammation. 2017. PMID: 29096654 Free PMC article.
-
Colocalization of aromatase in spinal cord astrocytes: differences in expression and relationship to mechanical and thermal hyperalgesia in murine models of a painful and a non-painful bone tumor.Neuroscience. 2015 Aug 20;301:235-45. doi: 10.1016/j.neuroscience.2015.06.009. Epub 2015 Jun 10. Neuroscience. 2015. PMID: 26071956 Free PMC article.
-
NFκB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice.J Neuroinflammation. 2014 Mar 1;11:38. doi: 10.1186/1742-2094-11-38. J Neuroinflammation. 2014. PMID: 24580964 Free PMC article.
References
-
- Sabino MA, Luger NM, Mach DB, Rogers SD, Schwei MJ, Mantyh PW. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer. 2003;104:550–558. doi: 10.1002/ijc.10999. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

