Encapsulation of alpha-1 antitrypsin in PLGA nanoparticles: in vitro characterization as an effective aerosol formulation in pulmonary diseases
- PMID: 22607686
- PMCID: PMC3485170
- DOI: 10.1186/1477-3155-10-20
Encapsulation of alpha-1 antitrypsin in PLGA nanoparticles: in vitro characterization as an effective aerosol formulation in pulmonary diseases
Abstract
Background: Alpha 1-antitrypsin (α1AT) belongs to the superfamily of serpins and inhibits different proteases. α1AT protects the lung from cellular inflammatory enzymes. In the absence of α1AT, the degradation of lung tissue results to pulmonary complications. The pulmonary route is a potent noninvasive route for systemic and local delivery. The aerosolized α1AT not only affects locally its main site of action but also avoids remaining in circulation for a long period of time in peripheral blood. Poly (D, L lactide-co glycolide) (PLGA) is a biodegradable and biocompatible polymer approved for sustained controlled release of peptides and proteins. The aim of this work was to prepare a wide range of particle size as a carrier of protein-loaded nanoparticles to deposit in different parts of the respiratory system especially in the deep lung. Various lactide to glycolide ratio of the copolymer was used to obtain different release profile of the drug which covers extended and rapid drug release in one formulation.
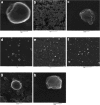
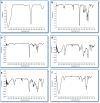
Results: Nonaqueous and double emulsion techniques were applied for the synthesis of nanoparticles. Nanoparticles were characterized in terms of surface morphology, size distribution, powder X-ray diffraction (XRD), encapsulation efficiency, in vitro drug release, FTIR spectroscopy and differential scanning calorimetry (DSC). To evaluate the nanoparticles cytotoxicity, cell cytotoxicity test was carried out on the Cor L105 human epithelial lung cancer cell line. Nanoparticles were spherical with an average size in the range of 100 nm to 1μ. The encapsulation efficiency was found to be higher when the double emulsion technique was applied. XRD and DSC results indicated that α1AT encapsulated in the nanoparticles existed in an amorphous or disordered-crystalline status in the polymer matrix. The lactic acid to glycolic acid ratio affects the release profile of α1AT. Hence, PLGA with a 50:50 ratios exhibited the ability to release %60 of the drug within 8, but the polymer with a ratio of 75:25 had a continuous and longer release profile. Cytotoxicity studies showed that nanoparticles do not affect cell growth and were not toxic to cells.
Conclusion: In summary, α1AT-loaded nanoparticles may be considered as a novel formulation for efficient treatment of many pulmonary diseases.
Figures






References
-
- Ugo I. EKEOWA, Bibek GOOPTU, Didier BELORGEY, Peter H¨AGGL ¨ OF, Susanna KARLSSON-LI, Elena MIRANDA, Juan P´EREZ, Ian MACLEOD, Heike KROGER, Stefan J. MARCINIAK, Damian C. CROWTHER and David A. LOMAS. α1-Antitrypsin deficiency, chronic obstructive pulmonary disease and the serpinopathies. Clinical Science. 2009;116:837–850. doi: 10.1042/CS20080484. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

