Nocturnin: at the crossroads of clocks and metabolism
- PMID: 22608110
- PMCID: PMC3389576
- DOI: 10.1016/j.tem.2012.03.007
Nocturnin: at the crossroads of clocks and metabolism
Abstract
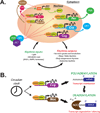
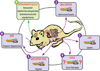
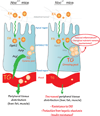
Many aspects of metabolism exhibit daily rhythmicity under the control of endogenous circadian clocks, and disruptions in circadian timing result in dysfunctions associated with the metabolic syndrome. Nocturnin (Noc) is a robustly rhythmic gene that encodes a deadenylase thought to be involved in the removal of polyA tails from mRNAs. Mice lacking the Noc gene display resistance to diet-induced obesity and hepatic steatosis, due in part to reduced lipid trafficking in the small intestine. In addition, Noc appears to play important roles in other tissues and has been implicated in lipid metabolism, adipogenesis, glucose homeostasis, inflammation and osteogenesis. Therefore, Noc is a potential key post-transcriptional mediator in the circadian control of many metabolic processes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
NOC out the fat: a short review of the circadian deadenylase Nocturnin.Ann Med. 2008;40(8):622-6. doi: 10.1080/07853890802084878. Ann Med. 2008. PMID: 18608124 Review.
-
Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity.Proc Natl Acad Sci U S A. 2007 Jun 5;104(23):9888-93. doi: 10.1073/pnas.0702448104. Epub 2007 May 17. Proc Natl Acad Sci U S A. 2007. PMID: 17517647 Free PMC article.
-
Nocturnin regulates circadian trafficking of dietary lipid in intestinal enterocytes.Curr Biol. 2011 Aug 23;21(16):1347-55. doi: 10.1016/j.cub.2011.07.018. Epub 2011 Aug 4. Curr Biol. 2011. PMID: 21820310 Free PMC article.
-
Posttranscriptional regulation of mammalian circadian clock output.Cold Spring Harb Symp Quant Biol. 2007;72:145-56. doi: 10.1101/sqb.2007.72.022. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419272 Review.
-
Spatiotemporal regulation of NADP(H) phosphatase Nocturnin and its role in oxidative stress response.Proc Natl Acad Sci U S A. 2020 Jan 14;117(2):993-999. doi: 10.1073/pnas.1913712117. Epub 2019 Dec 26. Proc Natl Acad Sci U S A. 2020. PMID: 31879354 Free PMC article.
Cited by
-
Circadian Posttranscriptional Regulatory Mechanisms in Mammals.Cold Spring Harb Perspect Biol. 2018 Jun 1;10(6):a030692. doi: 10.1101/cshperspect.a030692. Cold Spring Harb Perspect Biol. 2018. PMID: 28778869 Free PMC article. Review.
-
A Systems Biology Approach to Investigating Sex Differences in Cardiac Hypertrophy.J Am Heart Assoc. 2017 Aug 19;6(8):e005838. doi: 10.1161/JAHA.117.005838. J Am Heart Assoc. 2017. PMID: 28862954 Free PMC article.
-
Circadian physiology of metabolism.Science. 2016 Nov 25;354(6315):1008-1015. doi: 10.1126/science.aah4967. Science. 2016. PMID: 27885007 Free PMC article. Review.
-
Dim Light at Night Disturbs Molecular Pathways of Lipid Metabolism.Int J Mol Sci. 2020 Sep 21;21(18):6919. doi: 10.3390/ijms21186919. Int J Mol Sci. 2020. PMID: 32967195 Free PMC article.
-
Tumor necrosis factor and transforming growth factor β regulate clock genes by controlling the expression of the cold inducible RNA-binding protein (CIRBP).J Biol Chem. 2014 Jan 31;289(5):2736-44. doi: 10.1074/jbc.M113.508200. Epub 2013 Dec 11. J Biol Chem. 2014. PMID: 24337574 Free PMC article.
References
-
- Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. - PubMed
-
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

