Serum amyloid A triggers the mosodium urate -mediated mature interleukin-1β production from human synovial fibroblasts
- PMID: 22608202
- PMCID: PMC3446500
- DOI: 10.1186/ar3849
Serum amyloid A triggers the mosodium urate -mediated mature interleukin-1β production from human synovial fibroblasts
Abstract
Background: Monosodium urate (MSU) has been shown to promote inflammasome activation and interleukin-1β (IL-1β) secretion in monocyte/macrophages, but the cellular pathway and nod-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome activation in synovial tissues, remain elusive. In this study, we investigated the effects of MSU on synovial fibroblasts to elucidate the process of MSU-mediated synovial inflammation.
Methods: Human synovial fibroblasts were stimulated with MSU in the presence or absence of serum amyloid A (SAA). The cellular supernatants were analyzed by immunoblotting using anti-IL-1β or anti-caspase-1 antibodies. IL-1β or NLRP3 mRNA expressions were analyzed by real-time PCR or reverse transcription-PCR (RT-PCR) method.
Results: Neither SAA nor MSU stimulation resulted in IL-1β or interleukin-1α (IL-1α) secretions and pro-IL-1β processing in synovial fibroblasts. However, in SAA-primed synovial fibroblasts, MSU stimulation resulted in the activation of caspase-1 and production of active IL-1β and IL-1α. The effect of SAA on IL-1β induction was impaired in cells by silencing NLRP3 using siRNA or treating with caspase-1 inhibitor. In addition, SAA induced the secretion of cathepsin B and NLRP3 mRNA expression in synovial fibroblasts.
Conclusions: Our data demonstrate that exposure of human synovial fibroblasts to SAA promotes MSU-mediated caspase-1 activation and IL-1β secretion in the absence of microbial stimulation. These findings provide insight into the molecular processes underlying the synovial inflammatory condition of gout.
Figures
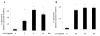
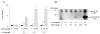
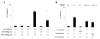
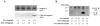


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

