Immunization targeting a minor plaque constituent clears β-amyloid and rescues behavioral deficits in an Alzheimer's disease mouse model
- PMID: 22608241
- PMCID: PMC3426627
- DOI: 10.1016/j.neurobiolaging.2012.04.007
Immunization targeting a minor plaque constituent clears β-amyloid and rescues behavioral deficits in an Alzheimer's disease mouse model
Abstract
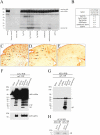
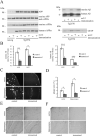
Although anti-human β-amyloid (Aβ) immunotherapy clears brain β-amyloid plaques in Alzheimer's disease (AD), targeting additional brain plaque constituents to promote clearance has not been attempted. Endogenous murine Aβ is a minor Aβ plaque component in amyloid precursor protein (APP) transgenic AD models, which we show is ∼3%-8% of the total accumulated Aβ in various human APP transgenic mice. Murine Aβ codeposits and colocalizes with human Aβ in amyloid plaques, and the two Aβ species coimmunoprecipitate together from brain extracts. In the human APP transgenic mouse model Tg2576, passive immunization for 8 weeks with a murine-Aβ-specific antibody reduced β-amyloid plaque pathology, robustly decreasing both murine and human Aβ levels. The immunized mice additionally showed improvements in two behavioral assays, odor habituation and nesting behavior. We conclude that passive anti-murine Aβ immunization clears Aβ plaque pathology--including the major human Aβ component--and decreases behavioral deficits, arguing that targeting minor endogenous brain plaque constituents can be beneficial, broadening the range of plaque-associated targets for AD therapeutics.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology. 2005;64(1):94–101. - PubMed
-
- Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA, Hutchinson WL, Mangione PP, Gallimore JR, Millar DJ, Minogue S, Dhillon AP, Taylor GW, Bradwell AR, Petrie A, Gillmore JD, Bellotti V, Botto M, Hawkins PN, Pepys MB. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468(7320):93–7. - PMC - PubMed
-
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276(24):21562–70. - PubMed
-
- Choi-Miura NH, Ihara Y, Fukuchi K, Takeda M, Nakano Y, Tobe T, Tomita M. SP-40,40 is a constituent of Alzheimer's amyloid. Acta neuropathologica. 1992;83(3):260–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

