Metformin: direct inhibition of rat ovarian theca-interstitial cell proliferation
- PMID: 22608319
- PMCID: PMC3389190
- DOI: 10.1016/j.fertnstert.2012.04.010
Metformin: direct inhibition of rat ovarian theca-interstitial cell proliferation
Abstract
Objective: To determine whether metformin has direct effects on ovarian theca-interstitial (T-I) cell proliferation through activation of adenosine monophosphate-activated protein kinase (AMPK).
Design: In vitro experimental study.
Setting: Academic medical center laboratory.
Animal(s): Immature Sprague-Dawley female rats.
Intervention(s): Ovarian T-I cells were isolated, purified, and cultured in the absence (control) or presence of insulin (1 μg/mL) with or without metformin or other activators/inhibitors of AMPK (AICAR, compound C).
Main outcome measure(s): Proliferation assessed by determination of expression levels of proteins involved in cell cycle progression, cyclin D3, and cyclin-dependent kinase 4 (CDK4) with Western blot analysis, and determination of DNA synthesis with bromodeoxyuridine (BrdU) incorporation assay; activation of AMPK, Erk1/2, and S6K1 determined by Western blot analysis with the use of antibodies specific for the phosphorylated (activated) forms.
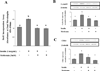
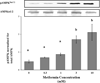
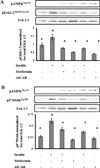
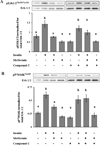
Result(s): Metformin inhibited insulin-induced ovarian T-I cell proliferation and the up-regulation of the cell cycle regulatory proteins, cyclin D3 and CDK4. Metformin independently activated AMPK in a dose-dependent manner. Treatment with metformin inhibited insulin-induced activation of Erk1/2 and S6K1. This effect was reversed with the addition of compound C, a known AMPK inhibitor.
Conclusion(s): Metformin directly inhibits proliferation of ovarian T-I cells via an AMPK-dependent mechanism. These findings further validate the potential benefits of metformin in the treatment of conditions associated with hyperinsulinemia and excessive growth of ovarian T-I cells (such as polycystic ovary syndrome).
Copyright © 2012 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Phosphorylation and activation of AMP-activated protein kinase (AMPK) by metformin in the human ovary requires insulin.Endocrinology. 2011 Mar;152(3):1112-8. doi: 10.1210/en.2009-1429. Epub 2011 Jan 5. Endocrinology. 2011. PMID: 21209024
-
Mevastatin inhibits proliferation of rat ovarian theca-interstitial cells by blocking the mitogen-activated protein kinase pathway.Fertil Steril. 2006 Oct;86(4 Suppl):1053-8. doi: 10.1016/j.fertnstert.2006.04.020. Epub 2006 Sep 11. Fertil Steril. 2006. PMID: 16963032
-
Zedoarondiol Inhibits Platelet-Derived Growth Factor-Induced Vascular Smooth Muscle Cells Proliferation via Regulating AMP-Activated Protein Kinase Signaling Pathway.Cell Physiol Biochem. 2016;40(6):1506-1520. doi: 10.1159/000453201. Epub 2016 Dec 21. Cell Physiol Biochem. 2016. PMID: 27997894
-
[Antitumor mechanism of metformin via adenosine monophosphate-activated protein kinase (AMPK) activation].Zhongguo Fei Ai Za Zhi. 2013 Aug 20;16(8):427-32. doi: 10.3779/j.issn.1009-3419.2013.08.07. Zhongguo Fei Ai Za Zhi. 2013. PMID: 23945247 Free PMC article. Review. Chinese.
-
Metformin as a Potential In Vitro Anticancer Modulator of Adenosine Monophosphate Kinase: A Review.Int J Breast Cancer. 2024 Aug 27;2024:1094274. doi: 10.1155/2024/1094274. eCollection 2024. Int J Breast Cancer. 2024. PMID: 39246697 Free PMC article. Review.
Cited by
-
Effect of berberine on insulin resistance in women with polycystic ovary syndrome: study protocol for a randomized multicenter controlled trial.Trials. 2013 Jul 18;14:226. doi: 10.1186/1745-6215-14-226. Trials. 2013. PMID: 23866924 Free PMC article. Clinical Trial.
-
Impact of metformin on reproductive tissues: an overview from gametogenesis to gestation.Ann Transl Med. 2014 Jun;2(6):55. doi: 10.3978/j.issn.2305-5839.2014.06.04. Ann Transl Med. 2014. PMID: 25333030 Free PMC article. Review.
-
Ovarian morphology is associated with insulin resistance in women with polycystic ovary syndrome: a cross sectional study.Fertil Res Pract. 2017 May 30;3:8. doi: 10.1186/s40738-017-0035-z. eCollection 2017. Fertil Res Pract. 2017. PMID: 28620546 Free PMC article.
-
hUCMSCs reduce theca interstitial cells apoptosis and restore ovarian function in premature ovarian insufficiency rats through regulating NR4A1-mediated mitochondrial mechanisms.Reprod Biol Endocrinol. 2022 Aug 19;20(1):125. doi: 10.1186/s12958-022-00992-5. Reprod Biol Endocrinol. 2022. PMID: 35986315 Free PMC article.
-
Effect of metformin on the human T98G glioblastoma multiforme cell line.Exp Ther Med. 2014 May;7(5):1285-1290. doi: 10.3892/etm.2014.1597. Epub 2014 Mar 4. Exp Ther Med. 2014. PMID: 24940426 Free PMC article.
References
-
- Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. - PubMed
-
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. - PubMed
-
- Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;326:1375–1379. - PubMed
-
- Longacre TA, Gilks CB. Nonneoplastic Lesions of the Ovary. In: Nucci MR, Oliva E, editors. Gynecologic Pathology, 1st ed. Orlando, Florida: Churchill Livingstone; 2009. pp. 367–391.
-
- Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79:1158–1165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

