Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of Lin(-)c-Kit(+) innate cell population
- PMID: 22608496
- PMCID: PMC3376903
- DOI: 10.1016/j.immuni.2012.03.021
Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of Lin(-)c-Kit(+) innate cell population
Abstract
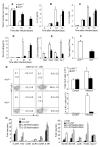
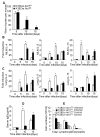
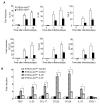
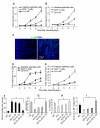
Interleukin-25 (IL-25 or IL-17E), a member of the structurally related IL-17 family, functions as an important mediator of T helper 2 cell-type (type 2) responses. We examined the cell type-specific role of IL-25-induced Act1-mediated signaling in protective immunity against helminth infection. Targeted Act1 deficiency in epithelial cells resulted in a marked delay in worm expulsion and abolished the expansion of the Lin(-)c-Kit(+) innate cell population in the mesenteric lymph node, lung, and liver. Th2 cell-inducing cytokine (IL-25 and IL-33) expression were reduced in the intestinal epithelial cells from the infected and IL-25-injected epithelial-specific Act1-deficient mice. Adoptive transfer of Lin(-)c-Kit(+) cells or combined injection of IL-25 and IL-33 restored the type 2 responses in these mice. Taken together, these results suggest that epithelial-specific Act1 mediates the expansion of the Lin(-)c-Kit(+) innate cell population through the positive-feedback loop of IL-25, initiating the type 2 immunity against helminth infection.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Innate immunity: Epithelial cells ACT up in worm expulsion.Nat Rev Immunol. 2012 Jun 15;12(7):475. doi: 10.1038/nri3249. Nat Rev Immunol. 2012. PMID: 22699829 No abstract available.
References
-
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. - PubMed
-
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

