Identification of a protein-containing enamel matrix layer which bridges with the dentine-enamel junction of adult human teeth
- PMID: 22609172
- PMCID: PMC3440526
- DOI: 10.1016/j.archoralbio.2012.04.014
Identification of a protein-containing enamel matrix layer which bridges with the dentine-enamel junction of adult human teeth
Abstract
Objective: To investigate the ultrastructure and chemical composition of the dentine-enamel junction and adjacent enamel of minimally processed third molar tooth sections.
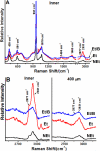
Design: Undecalcified human third molar erupted teeth were sectioned and etched with 4% EDTA or 37% phosphoric acid prior to visualization by scanning electron microscopy. Confocal Raman spectroscopy was carried out at 50 μm and more than 400 μm away from the dentine-enamel junction before and after mild etching.
Results: A novel organic protein-containing enamel matrix layer was identified for the first time using scanning electron microscopy of etched bucco-lingual sections of crowns. This layer resembles a three-dimensional fibrous meshwork that is visually distinct from enamel "tufts". Previous studies have generally used harsher solvent conditions which likely removed this layer and precluded its prior characterization. The shape of the organic enamel layer generally reflected that of sheath regions of enamel rods and extended from the dentine-enamel junction about 100-400 μm into the cuspal enamel. This layer exhibited a Raman CH stretching peak at ∼2931 cm(-1) characteristic of proteins and this signal correlated directly with the presence and location of the matrix layer as identified by scanning electron microscopy.
Conclusions: The enamel protein layer was most prominent close to the dentine-enamel junction and was largely absent in cuspal enamel >400 μm away from the dentine enamel junction. We hypothesize that this protein containing matrix layer could provide an important biomechanical linkage between the enamel and the dentine-enamel junction and by extension, with the dentine, of the adult tooth (246 words).
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Swine teeth as potential substitutes for in vitro studies in tooth adhesion: a SEM observation.Arch Oral Biol. 2006 Jul;51(7):548-51. doi: 10.1016/j.archoralbio.2006.01.009. Epub 2006 Mar 27. Arch Oral Biol. 2006. PMID: 16564493
-
Ultrastructural and immunocytochemical studies of enamel tufts in human permanent teeth.Arch Histol Cytol. 1992 May;55(2):179-90. doi: 10.1679/aohc.55.179. Arch Histol Cytol. 1992. PMID: 1497948
-
Radiotherapy effect on nano-mechanical properties and chemical composition of enamel and dentine.Arch Oral Biol. 2015 May;60(5):690-7. doi: 10.1016/j.archoralbio.2015.02.020. Epub 2015 Feb 27. Arch Oral Biol. 2015. PMID: 25766468 Free PMC article.
-
The wear and tear of teeth.Med Princ Pract. 2015;24 Suppl 1(Suppl 1):3-13. doi: 10.1159/000367976. Epub 2014 Nov 21. Med Princ Pract. 2015. PMID: 25427777 Free PMC article. Review.
-
Immunohistochemical and biochemical assay of versican in human sound predentine/dentine matrix.Eur J Histochem. 2009 Sep 23;53(3):e15. doi: 10.4081/ejh.2009.e15. Eur J Histochem. 2009. PMID: 19864206 Free PMC article. Review.
Cited by
-
Extracts of irradiated mature human tooth crowns contain MMP-20 protein and activity.J Dent. 2014 May;42(5):626-35. doi: 10.1016/j.jdent.2014.02.013. Epub 2014 Mar 4. J Dent. 2014. PMID: 24607847 Free PMC article.
-
Type IV collagen is a novel DEJ biomarker that is reduced by radiotherapy.J Dent Res. 2014 Oct;93(10):1028-34. doi: 10.1177/0022034514548221. Epub 2014 Aug 21. J Dent Res. 2014. PMID: 25146181 Free PMC article.
-
Dentin reactions to caries are misinterpreted by histological "gold standards".F1000Res. 2014 Jan 16;3:13. doi: 10.12688/f1000research.3-13.v1. eCollection 2014. F1000Res. 2014. PMID: 25469227 Free PMC article.
-
Enamel organic matrix: potential structural role in enamel and relationship to residual basement membrane constituents at the dentin enamel junction.Connect Tissue Res. 2014 Aug;55 Suppl 1(0 1):33-7. doi: 10.3109/03008207.2014.923883. Connect Tissue Res. 2014. PMID: 25158177 Free PMC article.
-
Oral cancer radiotherapy affects enamel microhardness and associated indentation pattern morphology.Clin Oral Investig. 2018 May;22(4):1795-1803. doi: 10.1007/s00784-017-2275-z. Epub 2017 Nov 18. Clin Oral Investig. 2018. PMID: 29151196 Free PMC article.
References
-
- Ryu OH, Fincham AG, Hu CC, Zhang C, Qian Q, Bartlett JD, et al. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res. 1999;78(3):743–750. - PubMed
-
- Llano E, Pendas AM, Knauper V, Sorsa T, Salo T, Salido E, et al. Identification and structural and functional characterization of human enamelysin (MMP-20) Biochemistry. 1997;36(49):15101–15108. - PubMed
-
- Simmer JP, Hu JC. Expression, structure, and function of enamel proteinases. Connect Tissue Res. 2002;43(2–3):441–449. - PubMed
-
- Paine ML, Zhu DH, Luo W, Bringas P, Jr, Goldberg M, White SN, et al. Enamel biomineralization defects result from alterations to amelogenin self-assembly. J Struct Biol. 2000;132(3):191–200. - PubMed
-
- Paine ML, Zhu DH, Luo W, Snead ML. Overexpression of TRAP in the enamel matrix does not alter the enamel structural hierarchy. Cells Tissues Organs. 2004;176(1–3):7–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

