Pulsed high-intensity-focused US and tissue plasminogen activator (TPA) versus TPA alone for thrombolysis of occluded bypass graft in swine
- PMID: 22609287
- PMCID: PMC3511867
- DOI: 10.1016/j.jvir.2012.04.001
Pulsed high-intensity-focused US and tissue plasminogen activator (TPA) versus TPA alone for thrombolysis of occluded bypass graft in swine
Abstract
Purpose: Prosthetic arteriovenous or arterial-arterial bypass grafts can thrombose and be resistant to revascularization. A thrombosed bypass graft model was created to evaluate the potential therapeutic enhancement and safety profile of pulsed high-intensity-focused ultrasound (pHIFU) on pharmaceutical thrombolysis.
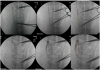
Materials and methods: In swine, a right carotid-carotid expanded polytetrafluoroethylene bypass graft was surgically constructed, containing a 40% stenosis at its distal end to induce graft thrombosis. The revascularization procedure was performed 7 days after surgery. After model development and dose response experiments (n = 11), two cohorts were studied: pHIFU with tissue plasminogen activator (TPA; n = 4) and sham pHIFU with TPA (n = 3). The experiments were identical in both groups except no energy was delivered in the sham pHIFU group. Serial angiograms were obtained in all cases. The area of graft opacified by contrast medium on angiograms was quantified with digital image processing software. A blinded reviewer calculated the change in the graft area opacified by contrast medium and expressed it as a percentage, representing percentage of thrombolysis.
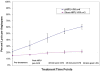
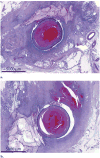
Results: Combining pHIFU with 0.5 mg of TPA resulted in a 52% ± 4% increase in thrombolysis on angiograms obtained at 30 minutes, compared with a 9% ± 14% increase with sham pHIFU and 0.5 mg TPA (P = .003). Histopathologic examination demonstrated no differences between the groups.
Conclusions: Thrombolysis of occluded bypass grafts was significantly increased when combining pHIFU and TPA versus sham pHIFU and TPA. These results suggest that application of pHIFU may augment thrombolysis with a reduced time and dose.
Copyright © 2012 SIR. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors have identified a conflict of interest.
Figures


Similar articles
-
Inertial Cavitation Ultrasound with Microbubbles Improves Reperfusion Efficacy When Combined with Tissue Plasminogen Activator in an In Vitro Model of Microvascular Obstruction.Ultrasound Med Biol. 2017 Jul;43(7):1391-1400. doi: 10.1016/j.ultrasmedbio.2017.02.013. Epub 2017 Apr 7. Ultrasound Med Biol. 2017. PMID: 28395964 Free PMC article.
-
Results of a prospective, randomized trial of surgery versus thrombolysis for occluded lower extremity bypass grafts.Am J Surg. 1996 Aug;172(2):105-12. doi: 10.1016/S0002-9610(96)00129-8. Am J Surg. 1996. PMID: 8795509 Clinical Trial.
-
The Enhancing Effect of Focused Ultrasound on TNK-Tissue Plasminogen Activator-Induced Thrombolysis Using an In Vitro Circulating Flow Model.J Stroke Cerebrovasc Dis. 2016 Dec;25(12):2891-2899. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.052. Epub 2016 Sep 2. J Stroke Cerebrovasc Dis. 2016. PMID: 27599905
-
Safety and efficacy of ultrasound-enhanced thrombolysis: a comprehensive review and meta-analysis of randomized and nonrandomized studies.Stroke. 2010 Feb;41(2):280-7. doi: 10.1161/STROKEAHA.109.563304. Epub 2009 Dec 31. Stroke. 2010. PMID: 20044531 Review.
-
Thrombolysis for restoration of patency to haemodialysis central venous catheters: a systematic review.J Thromb Thrombolysis. 2001 Apr;11(2):127-36. doi: 10.1023/a:1011272632286. J Thromb Thrombolysis. 2001. PMID: 11406727
Cited by
-
Design, characterization and evaluation of a laser-guided focused ultrasound system for preclinical investigations.Biomed Eng Online. 2019 Mar 28;18(1):36. doi: 10.1186/s12938-019-0656-z. Biomed Eng Online. 2019. PMID: 30922312 Free PMC article.
-
MR-guided transcranial focused ultrasound safely enhances interstitial dispersion of large polymeric nanoparticles in the living brain.PLoS One. 2018 Feb 7;13(2):e0192240. doi: 10.1371/journal.pone.0192240. eCollection 2018. PLoS One. 2018. PMID: 29415084 Free PMC article.
-
Focused Ultrasound: An Emerging Therapeutic Modality for Neurologic Disease.Neurotherapeutics. 2017 Apr;14(2):393-404. doi: 10.1007/s13311-017-0515-1. Neurotherapeutics. 2017. PMID: 28244011 Free PMC article.
References
-
- Fatourou EM, Paraskevas KI, Seifalian AM, Hamilton G, Mikhailidis DP. The role of established and emerging risk factors in peripheral vascular graft occlusion. Expert Opin Pharmacother. 2007;8:901–911. - PubMed
-
- Kapadia MR, Popowich DA, Kibbe MR. Modified prosthetic vascular conduits. Circulation. 2008;117:1873–1882. - PubMed
-
- Dormandy JA, Rutherford RB Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(suppl):S1–S296. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(suppl 1):S1–75. - PubMed
-
- Motarjeme A. Ultrasound-enhanced thrombolysis. J Endovasc Ther. 2007;14:251–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

