Bmi1 is required for regeneration of the exocrine pancreas in mice
- PMID: 22609312
- PMCID: PMC3485080
- DOI: 10.1053/j.gastro.2012.05.009
Bmi1 is required for regeneration of the exocrine pancreas in mice
Abstract
Background & aims: Bmi1 is a member of the Polycomb protein family and represses transcription by modifying chromatin organization at specific promoters. Bmi1 is implicated in the control of stem cell self-renewal and has been shown to regulate cell proliferation, tissue homeostasis, and differentiation. Bmi1 is present in a subpopulation of self-renewing pancreatic acinar cells and is expressed in response to pancreatic damage. We investigated the role of Bmi1 in regeneration of exocrine pancreas.
Methods: Acute pancreatitis was induced in Bmi1(-/-) mice with cerulein; pancreatic cell regeneration, differentiation, and apoptosis were assessed. Cultured Bmi1(-/-) and wild-type primary acini were analyzed in vitro to determine acinar-specific consequences of Bmi1 deletion. To investigate cell autonomous versus non-cell autonomous roles for Bmi1 in vivo, pancreatitis was induced in Bmi1(-/-) mice reconstituted with a wild-type hematopoietic system.
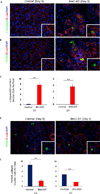
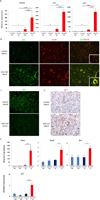
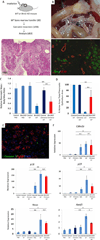
Results: Bmi1 expression was up-regulated in the exocrine pancreas during regeneration after cerulein-induced pancreatitis. Exocrine regeneration was impaired following administration of cerulein to Bmi1(-/-) mice. Pancreata of Bmi1(-/-) mice were hypoplastic, and the exocrine pancreas was replaced with ductal metaplasia that had increased apoptosis and decreased cell proliferation compared with that of wild-type mice. Expression of Cdkn2a and p53-dependent apoptotic genes was markedly up-regulated in Bmi1(-/-) pancreas compared with wild-type mice after injury. Furthermore, after transplantation of bone marrow from wild-type to Bmi1(-/-) mice, the chimeric mice had intermediate levels of pancreatic hypoplasia and significant but incomplete rescue of impaired exocrine regeneration after cerulein injury.
Conclusions: Bmi1 contributes to regeneration of the exocrine pancreas after cerulein-induced injury through cell autonomous mechanisms, in part by regulating Cdkn2a expression, and non-cell autonomous mechanisms.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
The epigenetic regulators Bmi1 and Ring1B are differentially regulated in pancreatitis and pancreatic ductal adenocarcinoma.J Pathol. 2009 Oct;219(2):205-13. doi: 10.1002/path.2585. J Pathol. 2009. PMID: 19585519
-
Loss of RE-1 silencing transcription factor accelerates exocrine damage from pancreatic injury.Cell Death Dis. 2020 Feb 20;11(2):138. doi: 10.1038/s41419-020-2269-7. Cell Death Dis. 2020. PMID: 32080178 Free PMC article.
-
Hedgehog signaling is required for effective regeneration of exocrine pancreas.Gastroenterology. 2008 Aug;135(2):621-31. doi: 10.1053/j.gastro.2008.04.011. Epub 2008 Apr 16. Gastroenterology. 2008. PMID: 18515092 Free PMC article.
-
BMI1 as a novel target for drug discovery in cancer.J Cell Biochem. 2011 Oct;112(10):2729-41. doi: 10.1002/jcb.23234. J Cell Biochem. 2011. PMID: 21678481 Review.
-
A protein synthesis brake for hematopoietic stem cell maintenance.Genes Dev. 2022 Aug 1;36(15-16):871-873. doi: 10.1101/gad.350107.122. Genes Dev. 2022. PMID: 36207141 Free PMC article. Review.
Cited by
-
Bmi1 Loss in the Organ of Corti Results in p16ink4a Upregulation and Reduced Cell Proliferation of Otic Progenitors In Vitro.PLoS One. 2016 Oct 18;11(10):e0164579. doi: 10.1371/journal.pone.0164579. eCollection 2016. PLoS One. 2016. PMID: 27755610 Free PMC article.
-
CBX7 silencing promoted liver regeneration by interacting with BMI1 and activating the Nrf2/ARE signaling pathway.Sci Rep. 2024 May 14;14(1):11008. doi: 10.1038/s41598-024-58248-8. Sci Rep. 2024. PMID: 38744845 Free PMC article.
-
Neutrophil Gelatinase-Associated Lipocalin Protects Acinar Cells From Cerulein-Induced Damage During Acute Pancreatitis.Pancreas. 2020 Nov/Dec;49(10):1297-1306. doi: 10.1097/MPA.0000000000001690. Pancreas. 2020. PMID: 33122517 Free PMC article.
-
Genetically engineered mouse models of pancreatic adenocarcinoma.Mol Oncol. 2013 Apr;7(2):232-47. doi: 10.1016/j.molonc.2013.02.002. Epub 2013 Feb 11. Mol Oncol. 2013. PMID: 23506980 Free PMC article. Review.
-
Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury.EBioMedicine. 2020 Aug;58:102920. doi: 10.1016/j.ebiom.2020.102920. Epub 2020 Jul 30. EBioMedicine. 2020. PMID: 32739869 Free PMC article.
References
-
- van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991;65:737–752. - PubMed
-
- Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991;65:753–763. - PubMed
-
- van Lohuizen M, Frasch M, Wientjens E, Berns A. Sequence similarity between the mammalian bmi-1 proto-oncogene and the Drosophila regulatory genes Psc and Su(z)2. Nature. 1991;353:353–355. - PubMed
-
- Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer. 2009;9:773–784. - PubMed
-
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

