The 21.5-kDa isoform of myelin basic protein has a non-traditional PY-nuclear-localization signal
- PMID: 22609403
- PMCID: PMC3526657
- DOI: 10.1016/j.bbrc.2012.05.051
The 21.5-kDa isoform of myelin basic protein has a non-traditional PY-nuclear-localization signal
Abstract
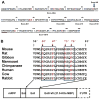
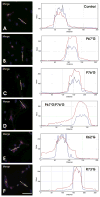
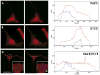
The predominant 18.5-kDa classic myelin basic protein (MBP) is mainly responsible for compaction of the myelin sheath in the central nervous system, but is multifunctional, having numerous interactions with Ca(2+)-calmodulin, actin, tubulin, and SH3-domains, and can tether these proteins to a lipid membrane in vitro. The full-length 21.5-kDa MBP isoform has an additional 26 residues encoded by exon-II of the classic gene, which causes it to be trafficked to the nucleus of oligodendrocytes (OLGs). We have performed site-directed mutagenesis of selected residues within this segment in red fluorescent protein (RFP)-tagged constructs, which were then transfected into the immortalized N19-OLG cell line to view protein localization using epifluorescence microscopy. We found that 21.5-kDa MBP contains two non-traditional PY-nuclear-localization signals, and that arginine and lysine residues within these motifs were involved in subcellular trafficking of this protein to the nucleus, where it may have functional roles during myelinogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Interaction of myelin basic protein with cytoskeletal and signaling proteins in cultured primary oligodendrocytes and N19 oligodendroglial cells.BMC Res Notes. 2014 Jun 24;7:387. doi: 10.1186/1756-0500-7-387. BMC Res Notes. 2014. PMID: 24956930 Free PMC article.
-
Classic 18.5- and 21.5-kDa myelin basic protein isoforms associate with cytoskeletal and SH3-domain proteins in the immortalized N19-oligodendroglial cell line stimulated by phorbol ester and IGF-1.Neurochem Res. 2012 Jun;37(6):1277-95. doi: 10.1007/s11064-011-0700-2. Epub 2012 Jan 17. Neurochem Res. 2012. PMID: 22249765 Free PMC article.
-
Nucleus-localized 21.5-kDa myelin basic protein promotes oligodendrocyte proliferation and enhances neurite outgrowth in coculture, unlike the plasma membrane-associated 18.5-kDa isoform.J Neurosci Res. 2013 Mar;91(3):349-62. doi: 10.1002/jnr.23166. Epub 2012 Nov 27. J Neurosci Res. 2013. PMID: 23184356 Free PMC article.
-
Myelin basic protein: a multifunctional protein.Cell Mol Life Sci. 2006 Sep;63(17):1945-61. doi: 10.1007/s00018-006-6094-7. Cell Mol Life Sci. 2006. PMID: 16794783 Free PMC article. Review.
-
Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms.J Neurochem. 2013 May;125(3):334-61. doi: 10.1111/jnc.12195. Epub 2013 Mar 6. J Neurochem. 2013. PMID: 23398367 Free PMC article. Review.
Cited by
-
Myelin Basic Protein and a Multiple Sclerosis-related MBP-peptide Bind to Oligonucleotides.Mol Ther Nucleic Acids. 2014 Sep 9;3(9):e192. doi: 10.1038/mtna.2014.43. Mol Ther Nucleic Acids. 2014. PMID: 25202925 Free PMC article.
-
Flexible Players within the Sheaths: The Intrinsically Disordered Proteins of Myelin in Health and Disease.Cells. 2020 Feb 18;9(2):470. doi: 10.3390/cells9020470. Cells. 2020. PMID: 32085570 Free PMC article. Review.
-
PA28γ-20S proteasome is a proteolytic complex committed to degrade unfolded proteins.Cell Mol Life Sci. 2021 Dec 16;79(1):45. doi: 10.1007/s00018-021-04045-9. Cell Mol Life Sci. 2021. PMID: 34913092 Free PMC article.
-
Impact of prenatal immune challenge on the demyelination injury during adulthood.CNS Neurosci Ther. 2017 Sep;23(9):724-735. doi: 10.1111/cns.12718. Epub 2017 Jul 17. CNS Neurosci Ther. 2017. PMID: 28718218 Free PMC article.
-
Comparative profiling of white matter development in the human and mouse brain reveals volumetric deficits and delayed myelination in Angelman syndrome.Res Sq [Preprint]. 2024 Aug 9:rs.3.rs-4681861. doi: 10.21203/rs.3.rs-4681861/v1. Res Sq. 2024. Update in: Mol Autism. 2024 Dec 26;15(1):54. doi: 10.1186/s13229-024-00636-y. PMID: 39149488 Free PMC article. Updated. Preprint.
References
-
- Bunge RP. Glial cells and the central myelin sheath. Physiol Rev. 1968;48:197–251. - PubMed
-
- Quarles RH, Macklin WB, Morell P. Myelin formation structure and biochemistry. In: Siegel GJ, Albers RW, Brady ST, Price DL, editors. Basic Neurochemistry–Molecular Cellular and Medical Aspects. Elsevier Academic Press; San Diego: 2006. pp. 51–71.
-
- Campagnoni AT, Pribyl TM, Campagnoni CW, Kampf K, Amur-Umarjee S, Landry CF, Handley VW, Newman SL, Garbay B, Kitamura K. Structure and developmental regulation of golli-mbp, a 105-kilobase gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocyte lineage in the brain. J Biol Chem. 1993;268:4930–4938. - PubMed
-
- Givogri MI, Bongarzone ER, Schonmann V, Campagnoni AT. Expression and regulation of golli products of myelin basic protein gene during in vitro development of oligodendrocytes. J Neurosci Res. 2001;66:679–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

