Beta1-adrenergic receptors promote focal adhesion signaling downregulation and myocyte apoptosis in acute volume overload
- PMID: 22609523
- PMCID: PMC3827977
- DOI: 10.1016/j.yjmcc.2012.05.004
Beta1-adrenergic receptors promote focal adhesion signaling downregulation and myocyte apoptosis in acute volume overload
Abstract
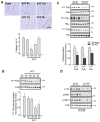
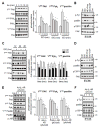
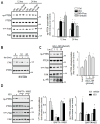
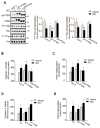
Numerous studies demonstrated increased expression of extracellular matrix (ECM) proteins and activation of focal adhesion (FA) signaling pathways in models of pressure overload-induced cardiac hypertrophy. However, little is known about FA signaling in response to volume overload where cardiac hypertrophy is associated with ECM loss. This study examines the role of beta1-adrenergic receptors (β(1)-ARs) in FA signaling changes and myocyte apoptosis induced during acute hemodynamic stress of volume overload. Rats with eccentric cardiac hypertrophy induced after aorto-caval fistula (ACF) develop reduced interstitial collagen content and decreased tyrosine phosphorylation of key FA signaling molecules FAK, Pyk(2) and paxillin along with an increase in cardiac myocyte apoptosis. ACF also increased activation of PTEN, a dual lipid and protein phosphatase, and its interaction with FA proteins. β(1)-AR blockade (extended-release of metoprolol succinate, 100mg QD) markedly attenuated PTEN activation, restored FA signaling and reduced myocyte apoptosis induced by ACF at 2days, but failed to reduce interstitial collagen loss and left ventricular dilatation. Treating cultured myocytes with β(1)-AR agonists or adenoviral expression of β(1)-ARs caused PTEN activation and interaction with FA proteins, thus leading to FA signaling downregulation and myocyte apoptosis. Adenoviral-mediated expression of a catalytically inactive PTEN mutant or wild-type FAK restored FA signaling downregulation and attenuated myocyte apoptosis induced by β(1)-ARs. Collectively, these data show that β(1)-AR stimulation in response to ACF induces FA signaling downregulation through an ECM-independent mechanism. This effect involves PTEN activation and may contribute to adverse cardiac remodeling and function in the course of volume overload.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None declared
Figures


Similar articles
-
Sympathetic activation causes focal adhesion signaling alteration in early compensated volume overload attributable to isolated mitral regurgitation in the dog.Circ Res. 2008 May 9;102(9):1127-36. doi: 10.1161/CIRCRESAHA.107.163642. Epub 2008 Mar 20. Circ Res. 2008. PMID: 18356543 Free PMC article.
-
Pleiotropic effects of neutrophils on myocyte apoptosis and left ventricular remodeling during early volume overload.J Mol Cell Cardiol. 2009 Nov;47(5):634-45. doi: 10.1016/j.yjmcc.2009.08.016. Epub 2009 Aug 28. J Mol Cell Cardiol. 2009. PMID: 19716828 Free PMC article.
-
Dynamic protein kinase a activities induced by beta-adrenoceptors dictate signaling propagation for substrate phosphorylation and myocyte contraction.Circ Res. 2009 Mar 27;104(6):770-9. doi: 10.1161/CIRCRESAHA.108.187880. Epub 2009 Feb 12. Circ Res. 2009. PMID: 19213958 Free PMC article.
-
Focal Adhesion's Role in Cardiomyocytes Function: From Cardiomyogenesis to Mechanotransduction.Cells. 2024 Apr 10;13(8):664. doi: 10.3390/cells13080664. Cells. 2024. PMID: 38667279 Free PMC article. Review.
-
Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance.Pharmacol Rev. 2013 Dec 24;66(1):308-33. doi: 10.1124/pr.112.007203. Print 2014. Pharmacol Rev. 2013. PMID: 24368739 Free PMC article. Review.
Cited by
-
Contributions of mechanical loading and hormonal changes to eccentric hypertrophy during volume overload: A Bayesian analysis using logic-based network models.PLoS Comput Biol. 2025 Apr 16;21(4):e1012390. doi: 10.1371/journal.pcbi.1012390. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40238825 Free PMC article.
-
Focal adhesion signaling in heart failure.Pflugers Arch. 2014 Jun;466(6):1101-11. doi: 10.1007/s00424-014-1456-8. Epub 2014 Feb 12. Pflugers Arch. 2014. PMID: 24515292 Free PMC article. Review.
-
CapZ and actin capping dynamics increase in myocytes after a bout of exercise and abates in hours after stimulation ends.J Appl Physiol (1985). 2013 Jun;114(11):1603-9. doi: 10.1152/japplphysiol.01283.2012. Epub 2013 Mar 14. J Appl Physiol (1985). 2013. PMID: 23493359 Free PMC article.
-
Effect of chronic pretreatment with beta-blockers on no-reflow phenomenon in diabetic patients with acute ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention.Egypt Heart J. 2017 Sep;69(3):171-175. doi: 10.1016/j.ehj.2017.01.001. Epub 2017 Apr 6. Egypt Heart J. 2017. PMID: 29622973 Free PMC article.
-
Signaling Pathways in Cardiac Myocyte Apoptosis.Biomed Res Int. 2016;2016:9583268. doi: 10.1155/2016/9583268. Epub 2016 Dec 22. Biomed Res Int. 2016. PMID: 28101515 Free PMC article. Review.
References
-
- Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol. 2001;33(5):887–905. - PubMed
-
- Steinberg SF. The molecular basis for distinct {beta}-adrenergic receptor subtype actions in cardiomyocytes. Circ Res. 1999;85(11):1101–11. - PubMed
-
- Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, et al. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101(14):1707–14. - PubMed
-
- Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, et al. Adverse effects of chronic endogenous sympathetic drive induced by cardiac Gs overexpression. Circ Res. 1996;78(4):517–24. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

