Role of cerebral endothelial cells in the astrocyte swelling and brain edema associated with acute hepatic encephalopathy
- PMID: 22609932
- PMCID: PMC4714767
- DOI: 10.1016/j.neuroscience.2012.05.006
Role of cerebral endothelial cells in the astrocyte swelling and brain edema associated with acute hepatic encephalopathy
Abstract
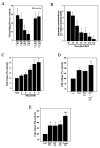
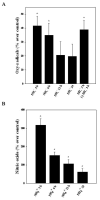
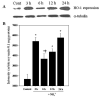
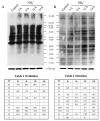
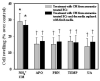
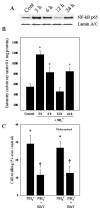
Brain edema is an important complication of acute hepatic encephalopathy (AHE), and astrocyte swelling is largely responsible for its development. Elevated blood and brain ammonia levels have been considered as major etiological factors in this edema. In addition to ammonia, recent studies have suggested that systemic infection, inflammation (and associated cytokines (CKs)), as well as endotoxin (lipopolysaccharide (LPS)) are also involved in AHE-associated brain edema. As endothelial cells (ECs) are the first resident brain cells exposed to blood-borne "noxious agents" (i.e., ammonia, CKs, LPS) that are present in AHE, these cells may be in a critical position to react to these agents and trigger a process resulting in astrocyte swelling/brain edema. We therefore examined the effect of conditioned media (CM) from ammonia, LPS and cytokine-treated cultured brain ECs on cell swelling in cultured astrocytes. CM from ammonia-treated ECs when added to astrocytes caused significant cell swelling, and such swelling was potentiated when astrocytes were exposed to CM from ECs treated with a combination of ammonia, LPS and CKs. We also found an additive effect when astrocytes were exposed to ammonia along with CM from ammonia-treated ECs. Additionally, ECs treated with ammonia showed a significant increase in the production of oxy-radicals, nitric oxide (NO), as well as evidence of oxidative/nitrative stress and activation of the transcription factor nuclear factor kappa B (NF-κB). CM derived from ECs treated with ammonia, along with antioxidants (AOs) or the NF-κB inhibitor BAY 11-7082, when added to astrocytes resulted in a significant reduction in cell swelling, as compared to the effect of CM from ECs-treated only with ammonia. We also identified increased nuclear NF-κB expression in rat brain cortical ECs in the thioacetamide (TAA) model of AHE. These studies suggest that ECs significantly contribute to the astrocyte swelling/brain edema in AHE, likely as a consequence of oxidative/nitrative stress and activation of NF-κB.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Albrecht J, Jones EA. Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci. 1999;170:138–146. - PubMed
-
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
-
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

