The FGF8-related signals Pyramus and Thisbe promote pathfinding, substrate adhesion, and survival of migrating longitudinal gut muscle founder cells
- PMID: 22609944
- PMCID: PMC3589571
- DOI: 10.1016/j.ydbio.2012.05.010
The FGF8-related signals Pyramus and Thisbe promote pathfinding, substrate adhesion, and survival of migrating longitudinal gut muscle founder cells
Abstract
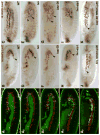
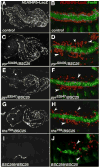
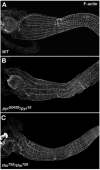
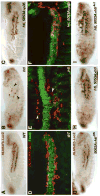
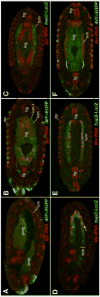
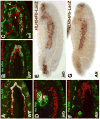
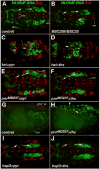
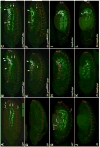
Fibroblast growth factors (FGFs) frequently fulfill prominent roles in the regulation of cell migration in various contexts. In Drosophila, the FGF8-like ligands Pyramus (Pyr) and Thisbe (Ths), which signal through their receptor Heartless (Htl), are known to regulate early mesodermal cell migration after gastrulation as well as glial cell migration during eye development. Herein, we show that Pyr and Ths also exert key roles during the long-distance migration of a specific sub-population of mesodermal cells that migrate from the caudal visceral mesoderm within stereotypic bilateral paths along the trunk visceral mesoderm toward the anterior. These cells constitute the founder myoblasts of the longitudinal midgut muscles. In a forward genetic screen for regulators of this morphogenetic process we identified loss of function alleles for pyr. We show that pyr and ths are expressed along the paths of migration in the trunk visceral mesoderm and endoderm and act largely redundantly to help guide the founder myoblasts reliably onto and along their substrate of migration. Ectopically-provided Pyr and Ths signals can efficiently re-rout the migrating cells, both in the presence and absence of endogenous signals. Our data indicate that the guidance functions of these FGFs must act in concert with other important attractive or adhesive activities of the trunk visceral mesoderm. Apart from their guidance functions, the Pyr and Ths signals play an obligatory role for the survival of the migrating cells. Without these signals, essentially all of these cells enter cell death and detach from the migration substrate during early migration. We present experiments that allowed us to dissect the roles of these FGFs as guidance cues versus trophic activities during the migration of the longitudinal visceral muscle founders.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Collective Migrations of Drosophila Embryonic Trunk and Caudal Mesoderm-Derived Muscle Precursor Cells.Genetics. 2020 Jun;215(2):297-322. doi: 10.1534/genetics.120.303258. Genetics. 2020. PMID: 32487692 Free PMC article. Review.
-
The role of FGF signaling in guiding coordinate movement of cell groups: guidance cue and cell adhesion regulator?Cell Adh Migr. 2012 Sep-Oct;6(5):397-403. doi: 10.4161/cam.21103. Epub 2012 Sep 1. Cell Adh Migr. 2012. PMID: 23076054 Free PMC article. Review.
-
Differential and overlapping functions of two closely related Drosophila FGF8-like growth factors in mesoderm development.Development. 2009 Jul;136(14):2393-402. doi: 10.1242/dev.035451. Epub 2009 Jun 10. Development. 2009. PMID: 19515694 Free PMC article.
-
Synchronous and symmetric migration of Drosophila caudal visceral mesoderm cells requires dual input by two FGF ligands.Development. 2012 Feb;139(4):699-708. doi: 10.1242/dev.068791. Epub 2012 Jan 4. Development. 2012. PMID: 22219352 Free PMC article.
-
FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation.Development. 2009 Mar;136(5):739-47. doi: 10.1242/dev.027904. Epub 2009 Jan 21. Development. 2009. PMID: 19158183 Free PMC article.
Cited by
-
Myotube migration to cover and shape the testis of Drosophila depends on Heartless, Cadherin/Catenin, and myosin II.Biol Open. 2017 Dec 15;6(12):1876-1888. doi: 10.1242/bio.025940. Biol Open. 2017. PMID: 29122742 Free PMC article.
-
Hematopoietic cell-derived RELMα regulates hookworm immunity through effects on macrophages.J Leukoc Biol. 2018 Oct;104(4):855-869. doi: 10.1002/JLB.4A0917-369RR. Epub 2018 Jul 10. J Leukoc Biol. 2018. PMID: 29992625 Free PMC article.
-
Collective Migrations of Drosophila Embryonic Trunk and Caudal Mesoderm-Derived Muscle Precursor Cells.Genetics. 2020 Jun;215(2):297-322. doi: 10.1534/genetics.120.303258. Genetics. 2020. PMID: 32487692 Free PMC article. Review.
-
Distinct genetic programs guide Drosophila circular and longitudinal visceral myoblast fusion.BMC Cell Biol. 2014 Jul 8;15:27. doi: 10.1186/1471-2121-15-27. BMC Cell Biol. 2014. PMID: 25000973 Free PMC article.
-
The role of FGF signaling in guiding coordinate movement of cell groups: guidance cue and cell adhesion regulator?Cell Adh Migr. 2012 Sep-Oct;6(5):397-403. doi: 10.4161/cam.21103. Epub 2012 Sep 1. Cell Adh Migr. 2012. PMID: 23076054 Free PMC article. Review.
References
-
- Azpiazu N, Frasch M. tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993;7:1325–1340. - PubMed
-
- Baker R, Schubiger G. Autonomous and nonautonomous Notch functions for embryonic muscle and epidermis development in Drosophila. Development. 1996;122:617–626. - PubMed
-
- Beiman M, Shilo B, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10:2993–3002. - PubMed
-
- Brückner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. - PubMed
-
- Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev Cell. 2005;9:831–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

