Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models
- PMID: 22610068
- PMCID: PMC3427471
- DOI: 10.1038/nn.3109
Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models
Abstract
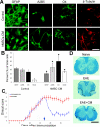
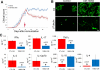
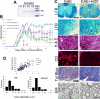
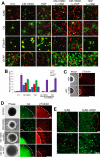
Mesenchymal stem cells (MSCs) have emerged as a potential therapy for a range of neural insults. In animal models of multiple sclerosis, an autoimmune disease that targets oligodendrocytes and myelin, treatment with human MSCs results in functional improvement that reflects both modulation of the immune response and myelin repair. Here we demonstrate that conditioned medium from human MSCs (MSC-CM) reduces functional deficits in mouse MOG35–55-induced experimental autoimmune encephalomyelitis (EAE) and promotes the development of oligodendrocytes and neurons. Functional assays identified hepatocyte growth factor (HGF) and its primary receptor cMet as critical in MSC-stimulated recovery in EAE, neural cell development and remyelination. Active MSC-CM contained HGF, and exogenously supplied HGF promoted recovery in EAE, whereas cMet and antibodies to HGF blocked the functional recovery mediated by HGF and MSC-CM. Systemic treatment with HGF markedly accelerated remyelination in lysolecithin-induced rat dorsal spinal cord lesions and in slice cultures. Together these data strongly implicate HGF in mediating MSC-stimulated functional recovery in animal models of multiple sclerosis.
Figures


References
-
- Prineas JW. The neuropathology of multiple sclerosis. In: Koestier JC, editor. Handbook of clinical neurology. Elsevier; Amsterdam: 1985.
-
- Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. New England Journal of Medicine. 1998;338:278–285. - PubMed
-
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. - PubMed
-
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. - PubMed
-
- Ben-Hur T, Goldman SA. Prospects of cell therapy for disorders of myelin. Annals of the New York Academy of Sciences. 2008;1142:218–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

