Cleavage of the NR2B subunit amino terminus of N-methyl-D-aspartate (NMDA) receptor by tissue plasminogen activator: identification of the cleavage site and characterization of ifenprodil and glycine affinities on truncated NMDA receptor
- PMID: 22610100
- PMCID: PMC3408171
- DOI: 10.1074/jbc.M112.374397
Cleavage of the NR2B subunit amino terminus of N-methyl-D-aspartate (NMDA) receptor by tissue plasminogen activator: identification of the cleavage site and characterization of ifenprodil and glycine affinities on truncated NMDA receptor
Abstract
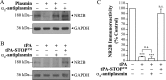
Thrombolysis using tissue plasminogen activator (tPA) has been the key treatment for patients with acute ischemic stroke for the past decade. Recent studies, however, suggest that this clot-busting protease also plays various roles in brain physiological and pathophysiological glutamatergic-dependent processes, such as synaptic plasticity and neurodegeneration. In addition, increasing evidence implicates tPA as an important neuromodulator of the N-methyl-d-aspartate (NMDA) receptors. Here, we demonstrate that recombinant human tPA cleaves the NR2B subunit of NMDA receptor. Analysis of NR2B in rat brain lysates and cortical neurons treated with tPA revealed concentration- and time-dependent degradation of NR2B proteins. Peptide sequencing studies performed on the cleaved-off products obtained from the tPA treatment on a recombinant fusion protein of the amino-terminal domain of NR2B revealed that tPA-mediated cleavage occurred at arginine 67 (Arg(67)). This cleavage is tPA-specific, plasmin-independent, and removes a predicted ~4-kDa fragment (Arg(27)-Arg(67)) from the amino-terminal domain of the NR2B protein. Site-directed mutagenesis of putative cleavage site Arg(67) to Ala(67) impeded tPA-mediated degradation of recombinant protein. This analysis revealed that NR2B is a novel substrate of tPA and suggested that an Arg(27)-Arg(67)-truncated NR2B-containing NMDA receptor could be formed. Heterologous expression of NR2B with Gln(29)-Arg(67) deleted is functional but exhibits reduced ifenprodil inhibition and increased glycine EC(50) with no change in glutamate EC(50). Our results confirmed NR2B as a novel proteolytic substrate of tPA, where tPA may directly interact with NR2B subunits leading to a change in pharmacological properties of NR2B-containing NMDA receptors.
Figures



References
-
- Collen D. (1999) The plasminogen (fibrinolytic) system. Thromb. Haemost. 82, 259–270 - PubMed
-
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N. Engl. J. Med. 333, 1581–1587 - PubMed
-
- Adams H. P., Jr., del Zoppo G., Alberts M. J., Bhatt D. L., Brass L., Furlan A., Grubb R. L., Higashida R. T., Jauch E. C., Kidwell C., Lyden P. D., Morgenstern L. B., Qureshi A. I., Rosenwasser R. H., Scott P. A., Wijdicks E. F., American Heart Association, American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups (2007) Guidelines for the early management of adults with ischemic stroke. A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38, 1655–1711 - PubMed
-
- Goldstein L. B. (2007) Acute ischemic stroke treatment in 2007. Circulation 116, 1504–1514 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

