Arrestin scaffolds NHERF1 to the P2Y12 receptor to regulate receptor internalization
- PMID: 22610101
- PMCID: PMC3397875
- DOI: 10.1074/jbc.M112.347104
Arrestin scaffolds NHERF1 to the P2Y12 receptor to regulate receptor internalization
Abstract
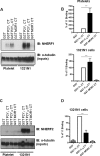
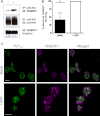
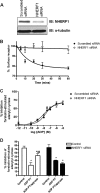
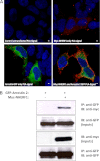
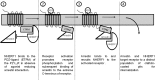
We have recently shown in a patient with mild bleeding that the PDZ-binding motif of the platelet G protein-coupled P2Y(12) receptor (P2Y(12)R) is required for effective receptor traffic in human platelets. In this study we show for the first time that the PDZ motif-binding protein NHERF1 exerts a major role in potentiating G protein-coupled receptor (GPCR) internalization. NHERF1 interacts with the C-tail of the P2Y(12)R and unlike many other GPCRs, NHERF1 interaction is required for effective P2Y(12)R internalization. In vitro and prior to agonist stimulation P2Y(12)R/NHERF1 interaction requires the intact PDZ binding motif of this receptor. Interestingly on receptor stimulation NHERF1 no longer interacts directly with the receptor but instead binds to the receptor via the endocytic scaffolding protein arrestin. These findings suggest a novel model by which arrestin can serve as an adaptor to promote NHERF1 interaction with a GPCR to facilitate effective NHERF1-dependent receptor internalization.
Figures


References
-
- Wolfe B. L., Trejo J. (2007) Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 8, 462–470 - PubMed
-
- Hanyaloglu A. C., von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 - PubMed
-
- Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature 401, 286–290 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

