Extended benefit from sequential administration of docetaxel after standard fluorouracil, epirubicin, and cyclophosphamide regimen for node-positive breast cancer: the 8-year follow-up results of the UNICANCER-PACS01 trial
- PMID: 22610153
- PMCID: PMC3399644
- DOI: 10.1634/theoncologist.2011-0442
Extended benefit from sequential administration of docetaxel after standard fluorouracil, epirubicin, and cyclophosphamide regimen for node-positive breast cancer: the 8-year follow-up results of the UNICANCER-PACS01 trial
Abstract
Purpose: The initial report from the Programme Action Concertée Sein (PACS) PACS01 trial demonstrated a benefit at 5 years for disease-free survival (DFS) and overall survival (OS) rates with the sequential administration of docetaxel after FEC100 (fluorouracil 500 mg/m(2), epirubicin 100 mg/m(2), and cyclophosphamide 500 mg/m(2)) for patients with node-positive, operable breast cancer. We evaluate here the impact of this regimen at 8 years.
Patients and methods: Between June 1997 and March 2000, a total of 1,999 patients (age <65) with localized, resectable, non-pretreated, unilateral breast cancer were randomly assigned to receive either standard FEC100 for 6 cycles or 3 cycles of FEC100 followed by 3 cycles of 100 mg/m(2) docetaxel (FEC-D), both given every 21 days. Radiotherapy was mandatory after conservative surgery and tamoxifen was given for 5 years to hormone receptor (HR)-positive patients. Five-year DFS was the trial's main endpoint. Updated 8-year survival data are presented.
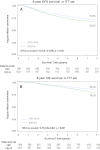
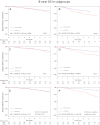
Results: With a median follow-up of 92.8 months, 639 patients experienced at least one event. A total number of 383 deaths were registered. Eight-year DFS rates were 65.8% with FEC alone and 70.2% with FEC-D. OS rates at 8 years were 78% with FEC alone and 83.2% with FEC-D. Cox regression analysis adjusted for age and number of positive nodes showed a 15% reduction in the relative risk of relapse and a 25% reduction in the relative risk of death in favor of FEC-D. Significant relative risk reductions were observed in the HR-positive, HER2-positive, and Ki67 ≥20% subpopulations.
Conclusion: Benefits for DFS and OS rates with the sequential FEC-D regimen are fully confirmed at 8 years.
Conflict of interest statement
Figures

References
-
- Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–5671. - PubMed
-
- Gianni L, Baselga J, Eiermann W, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol. 2009;27:2474–2481. - PubMed
-
- Martin M, Rodriguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. - PubMed
-
- De Laurentiis M, Cancello G, D'Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

