Dexamethasone blocks the systemic inflammation of alveolar hypoxia at several sites in the inflammatory cascade
- PMID: 22610172
- PMCID: PMC3404698
- DOI: 10.1152/ajpheart.00106.2012
Dexamethasone blocks the systemic inflammation of alveolar hypoxia at several sites in the inflammatory cascade
Abstract
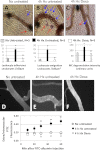
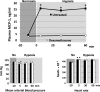
Alveolar hypoxia produces a rapid and widespread systemic inflammation in rats. The inflammation is initiated by the release into the circulation of monocyte chemoattractant protein-1 (MCP-1) from alveolar macrophages (AMO) activated by the low alveolar Po(2). Circulating MCP-1 induces mast cell (MC) degranulation with renin release and activation of the local renin-angiotensin system, leading to microvascular leukocyte recruitment and increased vascular permeability. We investigated the effect of dexamethasone, a synthetic anti-inflammatory glucocorticoid, on the development of the systemic inflammation of alveolar hypoxia and its site(s) of action in the inflammatory cascade. The inflammatory steps investigated were the activation of primary cultures of AMO by hypoxia, the degranulation of MCs by MCP-1 in the mesentery microcirculation of rats, and the effect of angiotensin II (ANG II) on the leukocyte/endothelial interface of the mesentery microcirculation. Dexamethasone prevented the mesentery inflammation in conscious rats breathing 10% O(2) for 4 h by acting in all key steps of the inflammatory cascade. Dexamethasone: 1) blocked the hypoxia-induced AMO activation and the release of MCP-1 and abolished the increase in plasma MCP-1 of conscious, hypoxic rats; 2) prevented the MCP-1-induced degranulation of mesentery perivascular MCs and reduced the number of peritoneal MCs, and 3) blocked the leukocyte-endothelial adherence and the extravasation of albumin induced by topical ANG II in the mesentery. The effect at each site was sufficient to prevent the AMO-initiated inflammation of hypoxia. These results may explain the effectiveness of dexamethasone in the treatment of the systemic effects of alveolar hypoxia.
Figures





Similar articles
-
Renin released from mast cells activated by circulating MCP-1 initiates the microvascular phase of the systemic inflammation of alveolar hypoxia.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2264-70. doi: 10.1152/ajpheart.00461.2011. Epub 2011 Sep 30. Am J Physiol Heart Circ Physiol. 2011. PMID: 21963836 Free PMC article.
-
The systemic inflammation of alveolar hypoxia is initiated by alveolar macrophage-borne mediator(s).Am J Respir Cell Mol Biol. 2009 Nov;41(5):573-82. doi: 10.1165/rcmb.2008-0417OC. Epub 2009 Feb 24. Am J Respir Cell Mol Biol. 2009. PMID: 19244200 Free PMC article.
-
Monocyte chemoattractant protein-1 released from alveolar macrophages mediates the systemic inflammation of acute alveolar hypoxia.Am J Respir Cell Mol Biol. 2011 Jul;45(1):53-61. doi: 10.1165/rcmb.2010-0264OC. Epub 2010 Sep 2. Am J Respir Cell Mol Biol. 2011. PMID: 20813992 Free PMC article.
-
Alveolar hypoxia-induced systemic inflammation: what low PO(2) does and does not do.Adv Exp Med Biol. 2010;662:27-32. doi: 10.1007/978-1-4419-1241-1_3. Adv Exp Med Biol. 2010. PMID: 20204767 Free PMC article. Review.
-
Alveolar hypoxia, alveolar macrophages, and systemic inflammation.Respir Res. 2009 Jun 22;10(1):54. doi: 10.1186/1465-9921-10-54. Respir Res. 2009. PMID: 19545431 Free PMC article. Review.
Cited by
-
Chronic Obstructive Pulmonary Disease and the Cardiovascular System: Vascular Repair and Regeneration as a Therapeutic Target.Front Cardiovasc Med. 2021 Apr 12;8:649512. doi: 10.3389/fcvm.2021.649512. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33912600 Free PMC article. Review.
-
Corticosteroids and perinatal hypoxic-ischemic brain injury.Drug Discov Today. 2018 Oct;23(10):1718-1732. doi: 10.1016/j.drudis.2018.05.019. Epub 2018 May 17. Drug Discov Today. 2018. PMID: 29778695 Free PMC article. Review.
-
The combined use of acetazolamide and Rhodiola in the prevention and treatment of altitude sickness.Ann Transl Med. 2022 May;10(10):541. doi: 10.21037/atm-22-2111. Ann Transl Med. 2022. PMID: 35722398 Free PMC article.
-
Bidirectional Crosstalk Between Hypoxia Inducible Factors and Glucocorticoid Signalling in Health and Disease.Front Immunol. 2021 Jun 4;12:684085. doi: 10.3389/fimmu.2021.684085. eCollection 2021. Front Immunol. 2021. PMID: 34149725 Free PMC article. Review.
-
Localized delivery of dexamethasone-21-phosphate via microdialysis implants in rat induces M(GC) macrophage polarization and alters CCL2 concentrations.Acta Biomater. 2015 Jan;12:11-20. doi: 10.1016/j.actbio.2014.10.022. Epub 2014 Oct 25. Acta Biomater. 2015. PMID: 25449921 Free PMC article.
References
-
- Beidleman BA, Muza SR, Fulco CS, Cymerman A, Staab JE, Sawka MN, Lewis SF, Skrinar GS. White blood cell, and hormonal responses to 4300 m altitude before and after intermittent altitude exposure. Clin Sci (Lond) 111: 163–169, 2006 - PubMed
-
- Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, Chung KF. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax 63: 784–790, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

