Crystal structure and mechanism of action of the N6-methyladenine-dependent type IIM restriction endonuclease R.DpnI
- PMID: 22610857
- PMCID: PMC3424567
- DOI: 10.1093/nar/gks428
Crystal structure and mechanism of action of the N6-methyladenine-dependent type IIM restriction endonuclease R.DpnI
Abstract
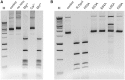
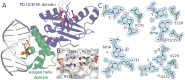
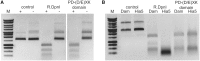
DNA methylation-dependent restriction enzymes have many applications in genetic engineering and in the analysis of the epigenetic state of eukaryotic genomes. Nevertheless, high-resolution structures have not yet been reported, and therefore mechanisms of DNA methylation-dependent cleavage are not understood. Here, we present a biochemical analysis and high-resolution DNA co-crystal structure of the N(6)-methyladenine (m6A)-dependent restriction enzyme R.DpnI. Our data show that R.DpnI consists of an N-terminal catalytic PD-(D/E)XK domain and a C-terminal winged helix (wH) domain. Surprisingly, both domains bind DNA in a sequence- and methylation-sensitive manner. The crystal contains R.DpnI with fully methylated target DNA bound to the wH domain, but distant from the catalytic domain. Independent readout of DNA sequence and methylation by the two domains might contribute to R.DpnI specificity or could help the monomeric enzyme to cut the second strand after introducing a nick.
Figures


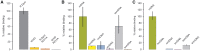


References
-
- Pingoud AM, editor. Restriction endonucleases. Berlin: Springer; 2004.
-
- Pieper U, Pingoud A. A mutational analysis of the PD … D/EXK motif suggests that McrC harbors the catalytic center for DNA cleavage by the GTP-dependent restriction enzyme McrBC from Escherichia coli. Biochemistry. 2002;41:5236–5244. - PubMed
-
- Bujnicki JM, Rychlewski L. Grouping together highly diverged PD-(D/E)XK nucleases and identification of novel superfamily members using structure-guided alignment of sequence profiles. J. Mol. Microbiol. Biotechnol. 2001;3:69–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

