Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging
- PMID: 22611035
- PMCID: PMC6870534
- DOI: 10.1002/hbm.22099
Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging
Abstract
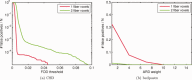
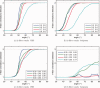
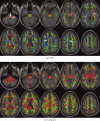
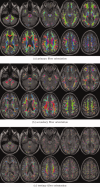
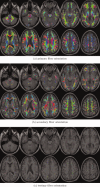
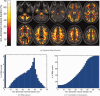
It has long been recognized that the diffusion tensor model is inappropriate to characterize complex fiber architecture, causing tensor-derived measures such as the primary eigenvector and fractional anisotropy to be unreliable or misleading in these regions. There is however still debate about the impact of this problem in practice. A recent study using a Bayesian automatic relevance detection (ARD) multicompartment model suggested that a third of white matter (WM) voxels contain crossing fibers, a value that, whilst already significant, is likely to be an underestimate. The aim of this study is to provide more robust estimates of the proportion of affected voxels, the number of fiber orientations within each WM voxel, and the impact on tensor-derived analyses, using large, high-quality diffusion-weighted data sets, with reconstruction parameters optimized specifically for this task. Two reconstruction algorithms were used: constrained spherical deconvolution (CSD), and the ARD method used in the previous study. We estimate the proportion of WM voxels containing crossing fibers to be ~90% (using CSD) and 63% (using ARD). Both these values are much higher than previously reported, strongly suggesting that the diffusion tensor model is inadequate in the vast majority of WM regions. This has serious implications for downstream processing applications that depend on this model, particularly tractography, and the interpretation of anisotropy and radial/axial diffusivity measures.
Keywords: bedpostx; constrained spherical deconvolution; crossing fibers; high-angular resolution diffusion imaging; partial volume effect; residual bootstrap; white matter.
Copyright © 2012 Wiley Periodicals, Inc.
Figures







References
-
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL (2001): Analysis of partial volume effects in diffusion‐tensor MRI. Magn Reson Med 45:770–780. - PubMed
-
- Alexander DC (2006): Multiple‐fiber reconstruction algorithms for diffusion MRI. Ann NY Acad Sci 1064:113–133. - PubMed
-
- Alexander DC, Barker GJ, Arridge SR (2002): Detection and modeling of non‐Gaussian apparent diffusion coefficient profiles in human brain data. Magn Reson Med 48:331–340. - PubMed
-
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. - PubMed
-
- Assaf Y, Pasternak O (2008): Diffusion tensor imaging (DTI)‐based white matter mapping in brain research: A review. J Mol Neurosci 34:51–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

