A p53 axis regulates B cell receptor-triggered, innate immune system-driven B cell clonal expansion
- PMID: 22611237
- PMCID: PMC3370112
- DOI: 10.4049/jimmunol.1103037
A p53 axis regulates B cell receptor-triggered, innate immune system-driven B cell clonal expansion
Abstract
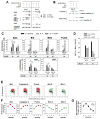
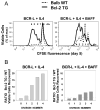
Resting mature human B cells undergo a dynamic process of clonal expansion, followed by clonal contraction, during an in vitro response to surrogate C3d-coated Ag and innate immune system cytokines, IL-4 and BAFF. In this study, we explore the mechanism for clonal contraction through following the time- and division-influenced expression of several pro- and anti-apoptotic proteins within CFSE-labeled cultures. Several findings, involving both human and mouse B cells, show that a mitochondria-dependent apoptotic pathway involving p53 contributes to the high activation-induced cell death (AICD) susceptibility of replicating blasts. Activated B cell clones exhibit elevated p53 protein and elevated mRNA/protein of proapoptotic molecules known to be under direct p53 transcriptional control, Bax, Bad, Puma, Bid, and procaspase 6, accompanied by reduced anti-apoptotic Bcl-2. Under these conditions, Bim levels were not increased. The finding that full-length Bid protein significantly declines in AICD-susceptible replicating blasts, whereas Bid mRNA does not, suggests that Bid is actively cleaved to short-lived, proapoptotic truncated Bid. AICD was diminished, albeit not eliminated, by p53 small interfering RNA transfection, genetic deletion of p53, or Bcl-2 overexpression. DNA damage is a likely trigger for p53-dependent AICD because susceptible lymphoblasts expressed significantly elevated levels of both phosphorylated ataxia telangiectasia mutated-Ser(1980) and phospho-H2AX-Ser(139). Deficiency in activation-induced cytosine deaminase diminishes but does not ablate murine B cell AICD, indicating that activation-induced cytosine deaminase-induced DNA damage is only in part responsible. Evidence for p53-influenced AICD during this route of T cell-independent clonal expansion raises the possibility that progeny bearing p53 mutations might undergo positive selection in peripherally inflamed tissues with elevated levels of IL-4 and BAFF.
Figures

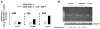

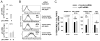



References
-
- El Shikh ME, El Sayed RM, Szakal AK, Tew JG. T-independent antibody responses to T-dependent antigens: a novel follicular dendritic cell-dependent activity. J Immunol. 2009;182:3482–3491. - PubMed
-
- Baumgarth N. A two-phase model of B-cell activation. Immunol Rev. 2000;176:171–180. - PubMed
-
- Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23:330–336. - PubMed
-
- Mongini PK, Inman JK, Han H, Fattah RJ, Abramson SB, Attur M. APRIL and BAFF promote increased viability of replicating human B2 cells via mechanism involving cyclooxygenase 2. J Immunol. 2006;176:6736–6751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

