Binuclear Cu(II) and Co(II) Complexes of Tridentate Heterocyclic Shiff Base Derived from Salicylaldehyde with 4-Aminoantipyrine
- PMID: 22611346
- PMCID: PMC3352244
- DOI: 10.1155/2012/601879
Binuclear Cu(II) and Co(II) Complexes of Tridentate Heterocyclic Shiff Base Derived from Salicylaldehyde with 4-Aminoantipyrine
Abstract
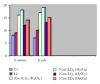
New binuclear Co(II) and Co(II) complexes of ONO tridentate heterocyclic Schiff base derived from 4-aminoantipyrine with salicylaldehyde have been synthesized and characterized on the bases of elemental analysis, UV-Vis., FT-IR, and also by aid of molar conductivity measurements, magnetic measurements, and melting points. It has been found that the Schiff bases with Cu(II) or Co(II) ion forming binuclear complexes on (1 : 1) "metal : ligand" stoichiometry. The molar conductance measurements of the complexes in DMSO correspond to be nonelectrolytic nature for all prepared complexes. Distorted octahedral environment is suggested for metal complexes. A theoretical treatment of the formation of complexes in the gas phase was studied, and this was done by using the HyperChem-6 program for the molecular mechanics and semi-empirical calculations. The free ligand and its complexes have been tested for their antibacterial activities against two types of human pathogenic bacteria: the first type (Staphylococcus aureus) is Gram positive and the second type (Escherichia coli) is Gram negative (by using agar well diffusion method). Finally, it was found that compounds show different activity of inhibition on growth of the bacteria.
Figures
References
-
- Garnovskii AD. Ambident chelating ligands. Zhurnal Neorganicheskoj Khimii. 1998;43(9):1491–1500.
-
- Sönmez M, Berber I, Akbaş E. Synthesis, antibacterial and antifungal activity of some new pyridazinone metal complexes. European Journal of Medicinal Chemistry. 2006;41(1):101–105. - PubMed
-
- Khalil SME, Seleem HS, El-Shetary BA, Shebl M. Mono- and bi-nuclear metal complexes of schiff-base hydrazone (ONN) derived from o-hydroxyacetophenone and 2-amino-4-hydrazino-6-methyl pyrimidine. Journal of Coordination Chemistry. 2002;55(8):883–899.
-
- Saleem SM, Hussain S, Nazir Z. Gastric teratoma—a rare benign tumour of neonates. Annals of Tropical Paediatrics. 2003;23(4):305–308. - PubMed
-
- Weitzer M, Brooker S. Bimetallic complexes of a structurally versatile pyridazine-containing Schiff-base macrocyclic ligand with pendant pyridine arms. Dalton Transactions. 2005;(14):2448–2454. - PubMed
LinkOut - more resources
Full Text Sources