Antiviral activities and putative identification of compounds in microbial extracts from the Hawaiian coastal waters
- PMID: 22611351
- PMCID: PMC3347012
- DOI: 10.3390/md10030521
Antiviral activities and putative identification of compounds in microbial extracts from the Hawaiian coastal waters
Abstract
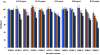
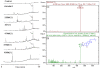
Marine environments are a rich source of significant bioactive compounds. The Hawaiian archipelago, located in the middle of the Pacific Ocean, hosts diverse microorganisms, including many endemic species. Thirty-eight microbial extracts from Hawaiian coastal waters were evaluated for their antiviral activity against four mammalian viruses including herpes simplex virus type one (HSV-1), vesicular stomatitis virus (VSV), vaccinia virus and poliovirus type one (poliovirus-1) using in vitro cell culture assay. Nine of the 38 microbial crude extracts showed antiviral potencies and three of these nine microbial extracts exhibited significant activity against the enveloped viruses. A secosteroid, 5α(H),17α(H),(20R)-beta-acetoxyergost-8(14)-ene was putatively identified and confirmed to be the active compound in these marine microbial extracts. These results warrant future in-depth tests on the isolation of these active elements in order to explore and validate their antiviral potential as important therapeutic remedies.
Keywords: marine extract; antiviral activity; antiviral drug; enveloped virus; secosteroids.
Figures


References
-
- Fenical W., Jensen P.R. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat. Chem. Biol. 2006;2:666–673. - PubMed
-
- Ausubel J.H., Crist D.T., Waggoner P.E. First Census of Marine Life 2010: Highlights of a Decade of Discovery. Census of Marine Life; Washington, DC, USA: 2010. p. 3.
-
- Faulkner D.J. Marine natural products. Nat. Prod. Rep. 2002;19:1–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

