Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte
- PMID: 22611397
- PMCID: PMC3348530
- DOI: 10.1155/2012/282574
Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte
Abstract
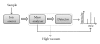
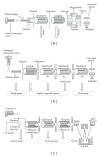
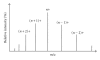
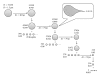
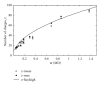
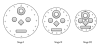
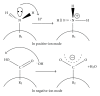
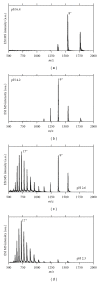
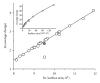
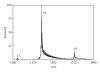
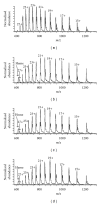
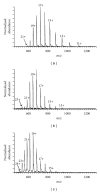
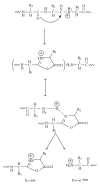
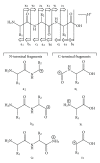
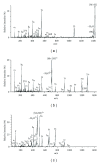
The Electrospray Ionization (ESI) is a soft ionization technique extensively used for production of gas phase ions (without fragmentation) of thermally labile large supramolecules. In the present review we have described the development of Electrospray Ionization mass spectrometry (ESI-MS) during the last 25 years in the study of various properties of different types of biological molecules. There have been extensive studies on the mechanism of formation of charged gaseous species by the ESI. Several groups have investigated the origin and implications of the multiple charge states of proteins observed in the ESI-mass spectra of the proteins. The charged analytes produced by ESI can be fragmented by activating them in the gas-phase, and thus tandem mass spectrometry has been developed, which provides very important insights on the structural properties of the molecule. The review will highlight recent developments and emerging directions in this fascinating area of research.
Figures







References
-
- Thomson JJ. Rays of Positive Electricity and Their Applications to Chemical Analysis. London, UK: Longmans Green; 1913.
-
- Grayson M. John Bennett Fenn: a curious road to the prize. Journal of The American Society for Mass Spectrometry. 2011;22(8):1301–1308. - PubMed
-
- Barber M, Bordoli RS, Sedgwick RD, Tyler AN. Fast atom bombardment of solids (F.A.B.): a new ion source for mass spectrometry. Journal of the Chemical Society, Chemical Communications. 1981;(7):325–327.
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246(4926):64–71. - PubMed
-
- Przybylski M, Glocker MO. Electrospray mass spectrometry of biomacromolecular complexes with noncovalent interactions—New analytical perspectives for supramolecular chemistry and molecular recognition processes. Angewandte Chemie. 1996;35(8):807–826.
LinkOut - more resources
Full Text Sources
Other Literature Sources

