Untethering the nuclear envelope and cytoskeleton: biologically distinct dystonias arising from a common cellular dysfunction
- PMID: 22611399
- PMCID: PMC3352338
- DOI: 10.1155/2012/634214
Untethering the nuclear envelope and cytoskeleton: biologically distinct dystonias arising from a common cellular dysfunction
Abstract
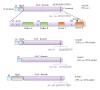
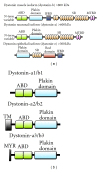
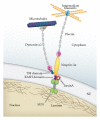
Most cases of early onset DYT1 dystonia in humans are caused by a GAG deletion in the TOR1A gene leading to loss of a glutamic acid (ΔE) in the torsinA protein, which underlies a movement disorder associated with neuronal dysfunction without apparent neurodegeneration. Mutation/deletion of the gene (Dst) encoding dystonin in mice results in a dystonic movement disorder termed dystonia musculorum, which resembles aspects of dystonia in humans. While torsinA and dystonin proteins do not share modular domain architecture, they participate in a similar function by modulating a structural link between the nuclear envelope and the cytoskeleton in neuronal cells. We suggest that through a shared interaction with the nuclear envelope protein nesprin-3α, torsinA and the neuronal dystonin-a2 isoform comprise a bridge complex between the outer nuclear membrane and the cytoskeleton, which is critical for some aspects of neuronal development and function. Elucidation of the overlapping roles of torsinA and dystonin-a2 in nuclear/endoplasmic reticulum dynamics should provide insights into the cellular mechanisms underlying the dystonic phenotype.
Figures
Similar articles
-
Biochemical and cellular analysis of human variants of the DYT1 dystonia protein, TorsinA/TOR1A.Hum Mutat. 2014 Sep;35(9):1101-13. doi: 10.1002/humu.22602. Epub 2014 Jul 17. Hum Mutat. 2014. PMID: 24930953 Free PMC article.
-
TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton.J Cell Sci. 2008 Oct 15;121(Pt 20):3476-86. doi: 10.1242/jcs.029454. Epub 2008 Sep 30. J Cell Sci. 2008. PMID: 18827015 Free PMC article.
-
TorsinA and dystonia: from nuclear envelope to synapse.J Neurochem. 2009 Jun;109(6):1596-609. doi: 10.1111/j.1471-4159.2009.06095.x. Epub 2009 Apr 8. J Neurochem. 2009. PMID: 19457118 Review.
-
The nuclear envelope localization of DYT1 dystonia torsinA-ΔE requires the SUN1 LINC complex component.BMC Cell Biol. 2011 May 31;12:24. doi: 10.1186/1471-2121-12-24. BMC Cell Biol. 2011. PMID: 21627841 Free PMC article.
-
The role of torsinA in dystonia.Eur J Neurol. 2010 Jul;17 Suppl 1:81-7. doi: 10.1111/j.1468-1331.2010.03057.x. Eur J Neurol. 2010. PMID: 20590813 Review.
Cited by
-
Mutations in the autoregulatory domain of β-tubulin 4a cause hereditary dystonia.Ann Neurol. 2013 Apr;73(4):546-53. doi: 10.1002/ana.23832. Epub 2013 Feb 19. Ann Neurol. 2013. PMID: 23424103 Free PMC article.
-
The genetics of dystonia: new twists in an old tale.Brain. 2013 Jul;136(Pt 7):2017-37. doi: 10.1093/brain/awt138. Epub 2013 Jun 17. Brain. 2013. PMID: 23775978 Free PMC article. Review.
-
The mutation responsible for torsion dystonia type 1 shows the ability to stimulate intracellular aggregation of mutant huntingtin.Dev Period Med. 2018;22(1):33-38. doi: 10.34763/devperiodmed.20182201.3338. Dev Period Med. 2018. PMID: 29641419 Free PMC article.
-
Biochemical and cellular analysis of human variants of the DYT1 dystonia protein, TorsinA/TOR1A.Hum Mutat. 2014 Sep;35(9):1101-13. doi: 10.1002/humu.22602. Epub 2014 Jul 17. Hum Mutat. 2014. PMID: 24930953 Free PMC article.
-
Regulation of Torsin ATPases by LAP1 and LULL1.Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):E1545-54. doi: 10.1073/pnas.1300676110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569223 Free PMC article.
References
-
- Fahn S. Concept and classification of dystonia. Advances in Neurology. 1988;50:1–8. - PubMed
-
- Geyer HL, Bressman SB. The diagnosis of dystonia. The Lancet Neurology. 2006;5(9):780–790. - PubMed
-
- Brüggemann N, Klein C. Genetics of primary torsion dystonia. Current Neurology and Neuroscience Reports. 2010;10(3):199–206. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

