Solanum lyratum Extracts Induce Extrinsic and Intrinsic Pathways of Apoptosis in WEHI-3 Murine Leukemia Cells and Inhibit Allograft Tumor
- PMID: 22611426
- PMCID: PMC3352350
- DOI: 10.1155/2012/254960
Solanum lyratum Extracts Induce Extrinsic and Intrinsic Pathways of Apoptosis in WEHI-3 Murine Leukemia Cells and Inhibit Allograft Tumor
Abstract
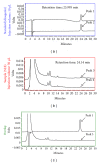
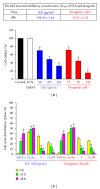
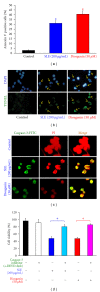
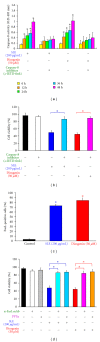
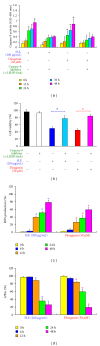
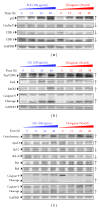
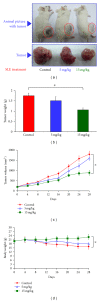
We investigated the molecular mechanisms of cell cycle arrest and apoptotic death induced by Solanum lyratum extracts (SLE) or diosgenin in WEHI-3 murine leukemia cells in vitro and antitumor activity in vivo. Diosgenin is one of the components of SLE. Our study showed that SLE and diosgenin decreased the viable WEHI-3 cells and induced G(0)/G(1) phase arrest and apoptosis in concentration- or time-dependent manners. Both reagents increased the levels of ROS production and decreased the mitochondrial membrane potential (ΔΨ(m)). SLE- and diosgenin-triggered apoptosis is mediated through modulating the extrinsic and intrinsic signaling pathways. Intriguingly, the p53 inhibitor (pifithrin-α), anti-Fas ligand (FasL) mAb, and specific inhibitors of caspase-8 (z-IETD-fmk), caspase-9 (z-LEHD-fmk), and caspase-3 (z-DEVD-fmk) blocked SLE- and diosgenin-reduced cell viability of WEHI-3 cells. The in vivo study demonstrated that SLE has marked antitumor efficacy against tumors in the WEHI-3 cell allograft model. In conclusion, SLE- and diosgenin-induced G(0)/G(1) phase arrest and triggered extrinsic and intrinsic apoptotic pathways via p53 activation in WEHI-3 cells. SLE also exhibited antitumor activity in vivo. Our findings showed that SLE may be potentially efficacious in the treatment of leukemia in the future.
Figures
References
-
- Lin JP, Yang JS, Lin JJ, et al. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo . Environmental Toxicology. 2011;13(4) - PubMed
-
- Sack H. Leukemia in patients with breast carcinoma after adjuvant chemotherapy and/or postoperative radiotherapy. Strahlentherapie Und Onkologie. 1995;171(7):420–421. - PubMed
-
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. - PubMed
-
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nature Reviews Molecular Cell Biology. 2007;8(5):405–413. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

