Studies on an alkali-thermostable xylanase from Aspergillus fumigatus MA28
- PMID: 22611527
- PMCID: PMC3339620
- DOI: 10.1007/s13205-011-0020-x
Studies on an alkali-thermostable xylanase from Aspergillus fumigatus MA28
Abstract
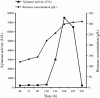
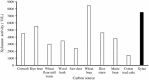
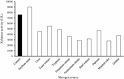
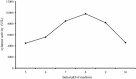
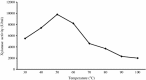
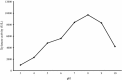
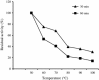
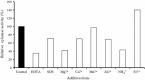
An alkalitolerant fungus, Aspergillus fumigatus strain MA28 produced significant amounts of cellulase-free xylanase when grown on a variety of agro-wastes. Wheat bran as the sole carbon source supported higher xylanase production (8,450 U/L) than xylan (7,500 U/L). Soybean meal was observed to be the best nitrogen source for xylanase production (9,000 U/L). Optimum medium pH for xylanase production was 8 (9,800 U/L), though, significant quantities of the enzyme was also produced at pH 7 (8,500 U/L), 9 (8,200 U/L) and 10 (4,600 U/L). The xylanase was purified by ammonium sulphate precipitation and carboxymethyl cellulose chromatography, and was found to have a molecular weight of 14.4 kDa with a V(max) of 980 μmol/min/mg of protein and a K(m) of approximately 4.9 mg/mL. The optimum temperature and pH for enzyme activity was 50 °C and pH 8, respectively. However, the enzyme also showed substantial residual activity at 60-70 °C (53-75%) and at alkaline pH 8-9 (56-88%).
Figures


References
-
- Badhan AK, Chadha BS, Saini HS. Purification of the alkaliphilic xylanases from Myceliophthora sp. IMI 387099 using cellulose-binding domain as an affinity tag. World J Microbiol Biotechnol. 2008;24:973–981. doi: 10.1007/s11274-007-9561-x. - DOI
-
- Bajaj BK, Sharma P (2011) An alkali-thermotolerant extracellular protease from a newly isolated Streptomyces sp. DP2. New Biotechnol. doi:10.1016/j.nbt.2011.01.001 - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

