Low proliferative potential and impaired angiogenesis of cultured rat kidney endothelial cells
- PMID: 22612333
- PMCID: PMC3458172
- DOI: 10.1111/j.1549-8719.2012.00193.x
Low proliferative potential and impaired angiogenesis of cultured rat kidney endothelial cells
Abstract
Objective: CKD is histologically characterized by interstitial fibrosis, which may be driven by peritubular capillary dropout and hypoxia. Surprisingly, peritubular capillaries have little repair capacity. We sought to establish long-term cultures of rat kidney endothelial cells to investigate their growth regulatory properties.
Methods: AKEC or YKEC were isolated using CD31-based isolation techniques and sustained in long-term cultures.
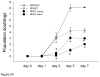
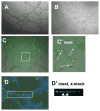
Results: Although YKEC grew slightly better than AKEC, both performed poorly compared with endothelial cells of the rat adult PMVEC, PAEC, or HUVEC cells. PMVEC and PAEC contained a large percentage of cells with high colony-forming potential. In contrast, KECs were incapable of forming large colonies and most remained as single nondividing cells. KEC expressed high levels of mRNA for VEGF receptors, but were surprisingly insensitive to VEGF stimulation. KEC did not form branching structures on Matrigel when cultured alone, but in mixed cultures, KEC incorporated into branching structures with PMVEC.
Conclusions: These data suggest that the intrinsic growth of rat kidney endothelial cells is limited by unknown mechanisms. The low growth rate may be related to the minimal intrinsic regenerative capacity of renal capillaries.
© 2012 John Wiley & Sons Ltd.
Figures








References
-
- Freeburg PB, Abrahamson DR. Hypoxia-Inducible Factors and Kidney Vascular Development. Journal of the American Society of Nephrology. 2003;14:2723–30. - PubMed
-
- Nyengaard J, Rasch R. The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia. 1993;36:189–94. - PubMed
-
- Nangaku M, Alpers CE, Pippin J, Shankland SJ, Adler S, Kurokawa K, Couser WG, Johnson RJ. A new model of renal microvascular endothelial injury. Kidney Int. 1997;52:182–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

