Maternal hypoxia and caffeine exposure depress fetal cardiovascular function during primary organogenesis
- PMID: 22612345
- PMCID: PMC3427416
- DOI: 10.1111/j.1447-0756.2012.01880.x
Maternal hypoxia and caffeine exposure depress fetal cardiovascular function during primary organogenesis
Abstract
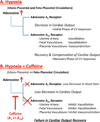
Aims: Hypoxia is known to influence cardiovascular (CV) function, in part, through adenosine receptor activation. We have shown in a mouse model that during primary cardiac morphogenesis, acute maternal hypoxia negatively affects fetal heart rate, and recurrent maternal caffeine exposure reduces fetal cardiac output (CO) and downregulates fetal adenosine A(2A) receptor gene expression. In the present study, we investigated whether maternal caffeine dosing exacerbates the fetal CV response to acute maternal hypoxia during the primary morphogenesis period.
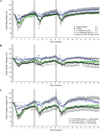
Material and methods: Gestational-day-11.5 pregnant mice were exposed to hypoxia (45 s duration followed by 10 min of recovery and repeated 3 times) while simultaneously monitoring maternal and fetal CO using high-resolution echocardiography.
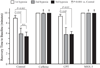
Results: Following maternal hypoxia exposure, maternal CO transiently decreased and then returned to pre-hypoxia baseline values. In contrast to a uniform maternal cardiac response to each exposure to hypoxia, the fetal CO recovery time to the baseline decreased, and CO rebounded above baseline following the second and third episodes of maternal hypoxia. Maternal caffeine treatment inhibited the fetal CO recovery to maternal hypoxia by lengthening the time to CO recovery and eliminating the CO rebound post-recovery. Selective treatment with an adenosine A(2A) receptor antagonist, but not an adenosine A(1) receptor antagonist, reproduced the altered fetal CO response to maternal hypoxia created by caffeine exposure.
Conclusions: Results suggest an additive negative effect of maternal caffeine on the fetal CV response to acute maternal hypoxia, potentially mediated via adenosine A(2A) receptor inhibition during primary cardiovascular morphogenesis.
© 2012 The Authors. Journal of Obstetrics and Gynaecology Research © 2012 Japan Society of Obstetrics and Gynecology.
Conflict of interest statement
There is no financial conflict of interest related to the current research.
Figures


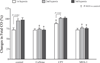
Similar articles
-
Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth.Am J Physiol Heart Circ Physiol. 2008 May;294(5):H2248-56. doi: 10.1152/ajpheart.91469.2007. Epub 2008 Mar 21. Am J Physiol Heart Circ Physiol. 2008. PMID: 18359892
-
Acute maternal and fetal cardiovascular effects of caffeine ingestion.Am J Perinatol. 1994 Mar;11(2):132-6. doi: 10.1055/s-2007-994573. Am J Perinatol. 1994. PMID: 8198655
-
The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists.Psychopharmacology (Berl). 2000 Feb;148(2):153-63. doi: 10.1007/s002130050037. Psychopharmacology (Berl). 2000. PMID: 10663430
-
Factors that increase reactivity during fetal nonstress testing.Curr Opin Obstet Gynecol. 2014 Apr;26(2):61-6. doi: 10.1097/GCO.0000000000000050. Curr Opin Obstet Gynecol. 2014. PMID: 24614020 Review.
-
Long-term consequences of disrupting adenosine signaling during embryonic development.Mol Aspects Med. 2017 Jun;55:110-117. doi: 10.1016/j.mam.2017.02.001. Epub 2017 Feb 13. Mol Aspects Med. 2017. PMID: 28202385 Free PMC article. Review.
Cited by
-
Caffeine and cardiovascular diseases: critical review of current research.Eur J Nutr. 2016 Jun;55(4):1331-43. doi: 10.1007/s00394-016-1179-z. Epub 2016 Mar 1. Eur J Nutr. 2016. PMID: 26932503 Review.
-
Evidence for ethnicity and location as regulators of the newborn blood metabolome: a monozygous twin study.Front Nutr. 2024 Jan 4;10:1259777. doi: 10.3389/fnut.2023.1259777. eCollection 2023. Front Nutr. 2024. PMID: 38239842 Free PMC article.
-
Validating the Paradigm That Biomechanical Forces Regulate Embryonic Cardiovascular Morphogenesis and Are Fundamental in the Etiology of Congenital Heart Disease.J Cardiovasc Dev Dis. 2020 Jun 12;7(2):23. doi: 10.3390/jcdd7020023. J Cardiovasc Dev Dis. 2020. PMID: 32545681 Free PMC article. Review.
-
Maternal Caffeine Consumption during Pregnancy and Risk of Low Birth Weight: A Dose-Response Meta-Analysis of Observational Studies.PLoS One. 2015 Jul 20;10(7):e0132334. doi: 10.1371/journal.pone.0132334. eCollection 2015. PLoS One. 2015. PMID: 26193706 Free PMC article.
References
-
- Keller BB. Function and biomechanics of developing cardiovascular systems. Formation of the Heart and Its Regulation. 2001
-
- Tobita KTJ, Keller BB. Cardiovascular Phenotype Analysis of Murine Embryos. In: HBaw RA, editor. Cardiovascular Physiology in the Genetically Engineered Mouse. 2nd Edition. New York: Kluwer Academic Publishers; 2002. pp. 353–376.
-
- Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clinical and experimental pharmacology & physiology. 2008;35(7):730–743. - PubMed
-
- Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2008;26(1):3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

