Fueling and imaging brain activation
- PMID: 22612861
- PMCID: PMC3401074
- DOI: 10.1042/AN20120021
Fueling and imaging brain activation
Erratum in
- ASN Neuro. 2012;4(6):423
Abstract
Metabolic signals are used for imaging and spectroscopic studies of brain function and disease and to elucidate the cellular basis of neuroenergetics. The major fuel for activated neurons and the models for neuron-astrocyte interactions have been controversial because discordant results are obtained in different experimental systems, some of which do not correspond to adult brain. In rats, the infrastructure to support the high energetic demands of adult brain is acquired during postnatal development and matures after weaning. The brain's capacity to supply and metabolize glucose and oxygen exceeds demand over a wide range of rates, and the hyperaemic response to functional activation is rapid. Oxidative metabolism provides most ATP, but glycolysis is frequently preferentially up-regulated during activation. Underestimation of glucose utilization rates with labelled glucose arises from increased lactate production, lactate diffusion via transporters and astrocytic gap junctions, and lactate release to blood and perivascular drainage. Increased pentose shunt pathway flux also causes label loss from C1 of glucose. Glucose analogues are used to assay cellular activities, but interpretation of results is uncertain due to insufficient characterization of transport and phosphorylation kinetics. Brain activation in subjects with low blood-lactate levels causes a brain-to-blood lactate gradient, with rapid lactate release. In contrast, lactate flooding of brain during physical activity or infusion provides an opportunistic, supplemental fuel. Available evidence indicates that lactate shuttling coupled to its local oxidation during activation is a small fraction of glucose oxidation. Developmental, experimental, and physiological context is critical for interpretation of metabolic studies in terms of theoretical models.
Figures


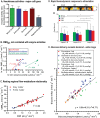

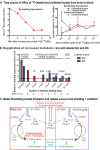

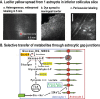
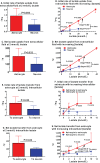

References
-
- Abe T, Takahashi S, Suzuki N. Oxidative metabolism in cultured rat astroglia: effects of reducing the glucose concentration in the culture medium and of D-aspartate or potassium stimulation. J Cereb Blood Flow Metab. 2006;26:153–160. - PubMed
-
- Ackermann RF, Lear JL. Glycolysis-induced discordance between glucose metabolic rates measured with radiolabeled fluorodeoxyglucose and glucose. J Cereb Blood Flow Metab. 1989;9:774–785. - PubMed
-
- Adachi K, Cruz NF, Sokoloff L, Dienel GA. Labeling of metabolic pools by [6–14C]glucose during K(+)-induced stimulation of glucose utilization in rat brain. J Cereb Blood Flow Metab. 1995;15:97–110. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
