The regulatory roles of apoptosis-inducing factor in the formation and regression processes of ocular neovascularization
- PMID: 22613025
- PMCID: PMC3388154
- DOI: 10.1016/j.ajpath.2012.03.022
The regulatory roles of apoptosis-inducing factor in the formation and regression processes of ocular neovascularization
Abstract
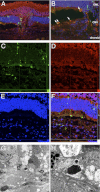
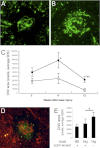
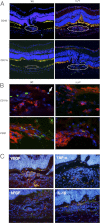
The role of apoptosis in the formation and regression of neovascularization is largely hypothesized, although the detailed mechanism remains unclear. Inflammatory cells and endothelial cells both participate and interact during neovascularization. During the early stage, these cells may migrate into an angiogenic site and form a pro-angiogenic microenvironment. Some angiogenic vessels appear to regress, whereas some vessels mature and remain. The control mechanisms of these processes, however, remain unknown. Previously, we reported that the prevention of mitochondrial apoptosis contributed to cellular survival via the prevention of the release of proapoptotic factors, such as apoptosis-inducing factor (AIF) and cytochrome c. In this study, we investigated the regulatory role of cellular apoptosis in angiogenesis using two models of ocular neovascularization: laser injury choroidal neovascularization and VEGF-induced corneal neovascularization in AIF-deficient mice. Averting apoptosis in AIF-deficient mice decreased apoptosis of leukocytes and endothelial cells compared to wild-type mice and resulted in the persistence of these cells at angiogenic sites in vitro and in vivo. Consequently, AIF deficiency expanded neovascularization and diminished vessel regression in these two models. We also observed that peritoneal macrophages from AIF-deficient mice showed anti-apoptotic survival compared to wild-type mice under conditions of starvation. Our data suggest that AIF-related apoptosis plays an important role in neovascularization and that mitochondria-regulated apoptosis could offer a new target for the treatment of pathological angiogenesis.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Retinal Inhibition of CCR3 Induces Retinal Cell Death in a Murine Model of Choroidal Neovascularization.PLoS One. 2016 Jun 16;11(6):e0157748. doi: 10.1371/journal.pone.0157748. eCollection 2016. PLoS One. 2016. PMID: 27309355 Free PMC article.
-
Isoliquiritigenin from licorice root suppressed neovascularisation in experimental ocular angiogenesis models.Br J Ophthalmol. 2011 Sep;95(9):1309-15. doi: 10.1136/bjophthalmol-2011-300110. Epub 2011 Jun 30. Br J Ophthalmol. 2011. PMID: 21719569
-
Endothelial TWIST1 promotes pathological ocular angiogenesis.Invest Ophthalmol Vis Sci. 2014 Nov 20;55(12):8267-77. doi: 10.1167/iovs.14-15623. Invest Ophthalmol Vis Sci. 2014. PMID: 25414194 Free PMC article.
-
Does apoptosis-inducing factor (AIF) have both life and death functions in cells?Bioessays. 2006 Aug;28(8):834-43. doi: 10.1002/bies.20444. Bioessays. 2006. PMID: 16927311 Review.
-
Life with or without AIF.Trends Biochem Sci. 2010 May;35(5):278-87. doi: 10.1016/j.tibs.2009.12.008. Epub 2010 Feb 6. Trends Biochem Sci. 2010. PMID: 20138767 Review.
Cited by
-
Gambogic acid induces apoptosis in diffuse large B-cell lymphoma cells via inducing proteasome inhibition.Sci Rep. 2015 Apr 8;5:9694. doi: 10.1038/srep09694. Sci Rep. 2015. PMID: 25853502 Free PMC article.
-
Corneal stromal wound healing: Major regulators and therapeutic targets.Ocul Surf. 2021 Jan;19:290-306. doi: 10.1016/j.jtos.2020.10.006. Epub 2020 Oct 28. Ocul Surf. 2021. PMID: 33127599 Free PMC article. Review.
-
VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway.Signal Transduct Target Ther. 2023 Aug 18;8(1):305. doi: 10.1038/s41392-023-01539-9. Signal Transduct Target Ther. 2023. PMID: 37591843 Free PMC article.
-
Gambogic acid induces apoptosis in imatinib-resistant chronic myeloid leukemia cells via inducing proteasome inhibition and caspase-dependent Bcr-Abl downregulation.Clin Cancer Res. 2014 Jan 1;20(1):151-63. doi: 10.1158/1078-0432.CCR-13-1063. Epub 2013 Dec 12. Clin Cancer Res. 2014. PMID: 24334603 Free PMC article.
-
Corneal angiogenic privilege and its failure.Exp Eye Res. 2021 Mar;204:108457. doi: 10.1016/j.exer.2021.108457. Epub 2021 Jan 22. Exp Eye Res. 2021. PMID: 33493471 Free PMC article. Review.
References
-
- Green D.R., Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. - PubMed
-
- Bao Q., Riedl S.J., Shi Y. Structure of Apaf-1 in the auto-inhibited form: a critical role for ADP. Cell Cycle. 2005;4:1001–1003. - PubMed
-
- Bao Q., Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. - PubMed
-
- Acehan D., Jiang X., Morgan D.G., Heuser J.E., Wang X., Akey C.W. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

