Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington's disease patient cells
- PMID: 22613578
- PMCID: PMC3506453
- DOI: 10.1186/1756-6606-5-17
Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington's disease patient cells
Abstract
Background: Huntington's Disease (HD) is a devastating neurodegenerative disorder that clinically manifests as motor dysfunction, cognitive impairment and psychiatric symptoms. There is currently no cure for this progressive and fatal disorder. The causative mutation of this hereditary disease is a trinucleotide repeat expansion (CAG) in the Huntingtin gene that results in an expanded polyglutamine tract. Multiple mechanisms have been proposed to explain the preferential striatal and cortical degeneration that occurs with HD, including non-cell-autonomous contribution from astrocytes. Although numerous cell culture and animal models exist, there is a great need for experimental systems that can more accurately replicate the human disease. Human induced pluripotent stem cells (iPSCs) are a remarkable new tool to study neurological disorders because this cell type can be derived from patients as a renewable, genetically tractable source for unlimited cells that are difficult to acquire, such as neurons and astrocytes. The development of experimental systems based on iPSC technology could aid in the identification of molecular lesions and therapeutic treatments.
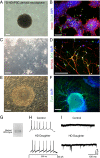
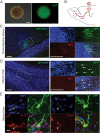
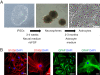
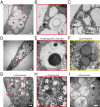
Results: We derived iPSCs from a father with adult onset HD and 50 CAG repeats (F-HD-iPSC) and his daughter with juvenile HD and 109 CAG repeats (D-HD-iPSC). These disease-specific iPSC lines were characterized by standard assays to assess the quality of iPSC lines and to demonstrate their pluripotency. HD-iPSCs were capable of producing phenotypically normal, functional neurons in vitro and were able to survive and differentiate into neurons in the adult mouse brain in vivo after transplantation. Surprisingly, when HD-iPSCs were directed to differentiate into an astrocytic lineage, we observed the presence of cytoplasmic, electron clear vacuoles in astrocytes from both F-HD-iPSCs and D-HD-iPSCs, which were significantly more pronounced in D-HD-astrocytes. Remarkably, the vacuolation in diseased astrocytes was observed under basal culture conditions without additional stressors and increased over time. Importantly, similar vacuolation phenotype has also been observed in peripheral blood lymphocytes from individuals with HD. Together, these data suggest that vacuolation may be a phenotype associated with HD.
Conclusions: We have generated a unique in vitro system to study HD pathogenesis using patient-specific iPSCs. The astrocytes derived from patient-specific iPSCs exhibit a vacuolation phenotype, a phenomenon previously documented in primary lymphocytes from HD patients. Our studies pave the way for future mechanistic investigations using human iPSCs to model HD and for high-throughput therapeutic screens.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

