Integrin αIIbβ3 inside-out activation: an in situ conformational analysis reveals a new mechanism
- PMID: 22613710
- PMCID: PMC3391112
- DOI: 10.1074/jbc.M112.360966
Integrin αIIbβ3 inside-out activation: an in situ conformational analysis reveals a new mechanism
Abstract
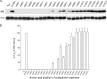
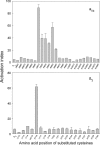
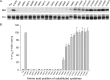
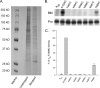
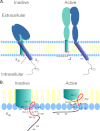
Integrins are a family of heterodimeric adhesion receptors that transmit signals bi-directionally across the plasma membranes. The transmembrane domain (TM) of integrin plays a critical role in mediating transition of the receptor from the default inactive to the active state on the cell surfaces. In this study, we successfully applied the substituted cysteine scanning accessibility method to determine the intracellular border of the integrin α(IIb)β(3) TM in the inactive and active states in living cells. We examined the aqueous accessibility of 75 substituted cysteines comprising the C terminus of both α(IIb) and β(3) TMs, the intracellular membrane-proximal regions, and the whole cytoplasmic tails, to the labeling of a membrane-permeable, cysteine-specific chemical biotin maleimide (BM). The active state of integrin α(IIb)β(3) heterodimer was generated by co-expression of activating partners with the cysteine-substituted constructs. Our data revealed that, in the inactive state, the intracellular lipid/aqueous border of α(IIb) TM was at Lys(994) and β(3) TM was at Phe(727) respectively; in the active state, the border of α(IIb) TM shifted to Pro(998), whereas the border of β(3) TM remained unchanged, suggesting that complex conformational changes occurred in the TMs upon α(IIb)β(3) inside-out activation. On the basis of the results, we propose a new inside-out activation mechanism for integrin α(IIb)β(3) and by inference, all of the integrins in their native cellular environment.
Figures



Similar articles
-
The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling.EMBO J. 2009 May 6;28(9):1351-61. doi: 10.1038/emboj.2009.63. Epub 2009 Mar 12. EMBO J. 2009. PMID: 19279667 Free PMC article.
-
Structural determinants of the integrin transmembrane domain required for bidirectional signal transmission across the cell membrane.J Biol Chem. 2021 Nov;297(5):101318. doi: 10.1016/j.jbc.2021.101318. Epub 2021 Oct 20. J Biol Chem. 2021. PMID: 34678312 Free PMC article.
-
Transmembrane domain helix packing stabilizes integrin alphaIIbbeta3 in the low affinity state.J Biol Chem. 2005 Feb 25;280(8):7294-300. doi: 10.1074/jbc.M412701200. Epub 2004 Dec 10. J Biol Chem. 2005. PMID: 15591321
-
Platelet integrin alpha(IIb)beta(3): activation mechanisms.J Thromb Haemost. 2007 Jul;5(7):1345-52. doi: 10.1111/j.1538-7836.2007.02537.x. J Thromb Haemost. 2007. PMID: 17635696 Review.
-
Clues for understanding the structure and function of a prototypic human integrin: the platelet glycoprotein IIb/IIIa complex.Thromb Haemost. 1994 Jul;72(1):1-15. Thromb Haemost. 1994. PMID: 7974356 Review.
Cited by
-
The dual structural roles of the membrane distal region of the α-integrin cytoplasmic tail during integrin inside-out activation.J Cell Sci. 2015 May 1;128(9):1718-31. doi: 10.1242/jcs.160663. Epub 2015 Mar 6. J Cell Sci. 2015. PMID: 25749862 Free PMC article.
-
Mechanisms of talin-dependent integrin signaling and crosstalk.Biochim Biophys Acta. 2014 Feb;1838(2):579-88. doi: 10.1016/j.bbamem.2013.07.017. Epub 2013 Jul 24. Biochim Biophys Acta. 2014. PMID: 23891718 Free PMC article. Review.
-
Talin-driven inside-out activation mechanism of platelet αIIbβ3 integrin probed by multimicrosecond, all-atom molecular dynamics simulations.Proteins. 2014 Dec;82(12):3231-3240. doi: 10.1002/prot.24540. Epub 2014 Sep 25. Proteins. 2014. PMID: 24677266 Free PMC article.
-
Is There Still a Role for Glycoprotein IIb/IIIa Antagonists in Acute Coronary Syndromes?Cardiol Res. 2013 Feb;4(1):1-7. doi: 10.4021/cr251w. Epub 2013 Mar 8. Cardiol Res. 2013. PMID: 28348696 Free PMC article. Review.
-
Integrin inactivators: balancing cellular functions in vitro and in vivo.Nat Rev Mol Cell Biol. 2013 Jul;14(7):430-42. doi: 10.1038/nrm3599. Epub 2013 May 30. Nat Rev Mol Cell Biol. 2013. PMID: 23719537 Review.
References
-
- Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

