Evidence that formation of vimentin mitogen-activated protein kinase (MAPK) complex mediates mast cell activation following FcεRI/CC chemokine receptor 1 cross-talk
- PMID: 22613718
- PMCID: PMC3397876
- DOI: 10.1074/jbc.M111.319624
Evidence that formation of vimentin mitogen-activated protein kinase (MAPK) complex mediates mast cell activation following FcεRI/CC chemokine receptor 1 cross-talk
Abstract
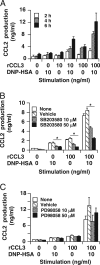
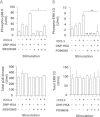
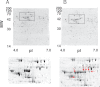
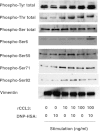
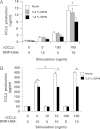
Accumulating evidence points to cross-talk between FcεRI and CC chemokine receptor (CCR)-mediated signaling pathways in mast cells. Here, we propose that vimentin, a protein comprising type III intermediate filament, participates in such cross-talk for CCL2/monocyte chemotactic protein 1 (MCP-1) production in mast cells, which is a mechanism for allergic inflammation. Co-stimulation via FcεRI, using IgE/antigen, and CCR1, using recombinant CCL3/macrophage inflammatory protein-1α (MIP-1α), increased expression of phosphorylated, disassembled, and soluble vimentin in rat basophilic leukemia (RBL)-2H3 cells expressing human CCR1 (RBL-CCR1 cells) and bone marrow-derived murine mast cells, both models of mucosal type mast cells. Furthermore, co-stimulation enhanced production of CCL2 as well as phosphorylation of MAPK. Treating the cells with p38 MAPK inhibitor SB203580, but not with MEK inhibitor PD98058, reduced CCL2 production, suggesting that p38 MAPK, but not ERK1/2, plays a critical role in the chemokine production. Immunoprecipitation analysis showed that vimentin interacts with phosphorylated ERK1/2 and p38 MAPKs in the co-simulated cells. Preventing disassembly of the vimentin by aggregating vimentin filaments using β,β'-iminodipropionitrile reduced the interaction of vimentin with phosphorylated MAPKs as well as CCL2 production in the cells. Taken together, disassembled vimentin interacting with phosphorylated p38 MAPK could mediate CCL2 production in mast cells upon FcεRI and CCR1 activation.
Figures

References
-
- Abramson J., Pecht I. (2007) Regulation of the mast cell response to the type 1 Fc ϵ receptor. Immunol. Rev. 217, 231–254 - PubMed
-
- Gilfillan A. M., Tkaczyk C. (2006) Integrated signaling pathways for mast cell activation. Nat. Rev. Immunol. 6, 218–230 - PubMed
-
- Ono S. J., Nakamura T., Miyazaki D., Ohbayashi M., Dawson M., Toda M. (2003) Chemokines. Roles in leukocyte development, trafficking, and effector function. J. Allergy Clin. Immunol. 111, 1185–1199; quiz 1200 - PubMed
-
- Beer F., Kuo C. H., Morohoshi K., Goodliffe J., Munro P., Aye C. C., Dawson M., Richardson R. M., Jones L. H., Ikeda Y., Nakamura T., Toda M., Flynn T., Ohbayashi M., Miyazaki D., Ono S. J. (2007) Role of β-chemokines in mast cell activation and type I hypersensitivity reactions in the conjunctiva. In vivo and in vitro studies. Immunol. Rev. 217, 96–104 - PubMed
-
- Tominaga T., Miyazaki D., Sasaki S., Mihara S., Komatsu N., Yakura K., Inoue Y. (2009) Blocking mast cell-mediated type I hypersensitivity in experimental allergic conjunctivitis by monocyte chemoattractant protein-1/CCR2. Invest. Ophthalmol. Vis. Sci. 50, 5181–5188 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

