Monod-Wyman-Changeux allosteric mechanisms of action and the pharmacology of etomidate
- PMID: 22614249
- PMCID: PMC3673006
- DOI: 10.1097/ACO.0b013e328354feea
Monod-Wyman-Changeux allosteric mechanisms of action and the pharmacology of etomidate
Abstract
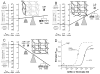
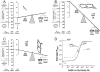
Purpose of review: Formal Monod-Wyman-Changeux allosteric mechanisms have proven valuable in framing research on the mechanism of etomidate action on its major molecular targets, γ-aminobutyric acid type A (GABAA) receptors. However, the mathematical formalism of these mechanisms makes them difficult to comprehend.
Recent findings: We illustrate how allosteric models represent shifting equilibria between various functional receptor states (closed versus open) and how co-agonism can be readily understood as simply addition of gating energy associated with occupation of distinct agonist sites. We use these models to illustrate how the functional effects of a point mutation, α1M236W, in GABAA receptors can be translated into an allosteric model phenotype.
Summary: Allosteric co-agonism provides a robust framework for design and interpretation of structure-function experiments aimed at understanding where and how etomidate affects its GABAA receptor target molecules.
Conflict of interest statement
The author has no conflicts of interest related to the content of this work. The author receives support from NIH grants (P01GM58448 and R01GM089745) for basic research, some of which is described in this work. The author is an inventor on patents for general anesthetic drugs that are not mentioned in this work. These patents are owned by the author’s employer, Massachusetts General Hospital, and the author has received no royalty payments.
Figures

Similar articles
-
Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2L GABA A receptors accounts for both direct activation and agonist modulation.J Biol Chem. 2004 May 14;279(20):20982-92. doi: 10.1074/jbc.M400472200. Epub 2004 Mar 11. J Biol Chem. 2004. PMID: 15016806
-
State-dependent etomidate occupancy of its allosteric agonist sites measured in a cysteine-substituted GABAA receptor.Mol Pharmacol. 2013 Jun;83(6):1200-8. doi: 10.1124/mol.112.084558. Epub 2013 Mar 22. Mol Pharmacol. 2013. PMID: 23525330 Free PMC article.
-
Correction for Inhibition Leads to an Allosteric Co-Agonist Model for Pentobarbital Modulation and Activation of α1β3γ2L GABAA Receptors.PLoS One. 2016 Apr 25;11(4):e0154031. doi: 10.1371/journal.pone.0154031. eCollection 2016. PLoS One. 2016. PMID: 27110714 Free PMC article.
-
Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels.Can J Anaesth. 2011 Feb;58(2):191-205. doi: 10.1007/s12630-010-9419-9. Epub 2011 Jan 7. Can J Anaesth. 2011. PMID: 21213095 Free PMC article. Review.
-
GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation.Can J Anaesth. 2011 Feb;58(2):206-15. doi: 10.1007/s12630-010-9429-7. Epub 2010 Dec 31. Can J Anaesth. 2011. PMID: 21194017 Free PMC article. Review.
Cited by
-
Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor.J Biol Chem. 2013 Jul 5;288(27):19343-57. doi: 10.1074/jbc.M113.479725. Epub 2013 May 15. J Biol Chem. 2013. PMID: 23677991 Free PMC article.
-
Activation of the α1β2γ2L GABAA Receptor by Physiological Agonists.Biomolecules. 2021 Dec 11;11(12):1864. doi: 10.3390/biom11121864. Biomolecules. 2021. PMID: 34944508 Free PMC article.
-
Intrasubunit and Intersubunit Steroid Binding Sites Independently and Additively Mediate α1β2γ2L GABAA Receptor Potentiation by the Endogenous Neurosteroid Allopregnanolone.Mol Pharmacol. 2021 Jul;100(1):19-31. doi: 10.1124/molpharm.121.000268. Epub 2021 May 6. Mol Pharmacol. 2021. PMID: 33958479 Free PMC article.
-
Activation of the Rat α1β2ε GABAA Receptor by Orthosteric and Allosteric Agonists.Biomolecules. 2022 Jun 21;12(7):868. doi: 10.3390/biom12070868. Biomolecules. 2022. PMID: 35883422 Free PMC article.
-
The Actions of Drug Combinations on the GABAA Receptor Manifest as Curvilinear Isoboles of Additivity.Mol Pharmacol. 2017 Nov;92(5):556-563. doi: 10.1124/mol.117.109595. Epub 2017 Aug 8. Mol Pharmacol. 2017. PMID: 28790148 Free PMC article.
References
-
- Franks NP, Lieb WR. Is membrane expansion relevant to anaesthesia? Nature. 1981;292(5820):248–51. - PubMed
-
- Franks NP, Lieb WR. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984;310(5978):599–601. - PubMed
-
- Grasshoff C, Rudolph U, Antkowiak B. Molecular and systemic mechanisms of general anaesthesia: the ‘multi-site and multiple mechanisms’ concept. Current Opinion in Anaesthesiology. 2005;18(4):386–91. - PubMed
-
- Solt K, Forman SA. Correlating the clinical actions and molecular mechanisms of general anesthetics. Current Opinion in Anaesthesiology. 2007;20(4):300–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

