Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load
- PMID: 22614889
- PMCID: PMC3881435
- DOI: 10.1097/QAD.0b013e328355e6b2
Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load
Abstract
Objective: To characterize HIV-infected patients with neurosymptomatic cerebrospinal fluid (CSF) 'escape', defined as detectable CSF HIV RNA in the setting of treatment-suppressed plasma levels or CSF RNA more than 1-log higher than plasma RNA.
Design: Retrospective case series.
Setting: Four urban medical centers in the United States and Europe.
Participants: Virologically controlled HIV-infected patients on antiretroviral therapy (ART) with progressive neurologic abnormalities who were determined to have CSF 'escape'. INTERVENTION Optimization of ART based upon drug susceptibility and presumed central nervous system exposure.
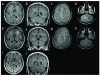
Main outcome measures: Levels of CSF HIV RNA and inflammatory markers, clinical signs and symptoms, and MRI findings.
Results: Ten patients presented with new neurologic abnormalities, which included sensory, motor, and cognitive manifestations. Median CSF HIV RNA was 3900 copies/ml (range 134-9056), whereas median plasma HIV RNA was 62 copies/ml (range <50 to 380). Median CD4 T-cell count was 482 cells/μl (range 290-660). All patients had been controlled to less than 500 copies/ml for median 27.5 months (range 2-96) and five of 10 had been suppressed to less than 50 copies/ml for median 19.5 months (range 2-96). Patients had documentation of a stable ART regimen for median 21 months (range 9-60). All had CSF pleocytosis or elevated CSF protein; seven of eight had abnormalities on MRI; and six of seven harbored CSF resistance mutations. Following optimization of ART, eight of nine patients improved clinically.
Conclusion: The development of neurologic symptoms in patients on ART with low or undetectable plasma HIV levels may be an indication of CSF 'escape'. This study adds to a growing body of literature regarding this rare condition in well controlled HIV infection.
Figures

Comment in
-
Symptomatic cerebrospinal fluid HIV escape syndrome in a patient on highly active antiretroviral therapy and suppressed plasma viral load.AIDS. 2019 Dec 1;33(15):2444-2446. doi: 10.1097/QAD.0000000000002370. AIDS. 2019. PMID: 31764112 No abstract available.
References
-
- Gisslen M, Fuchs D, Svennerholm B, Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Defic Syndr. 1999;21:271–6. - PubMed
-
- Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

