Benefits and adverse events in younger versus older patients receiving adjuvant chemotherapy for colon cancer: findings from the Adjuvant Colon Cancer Endpoints data set
- PMID: 22614981
- PMCID: PMC3675692
- DOI: 10.1200/JCO.2011.41.1975
Benefits and adverse events in younger versus older patients receiving adjuvant chemotherapy for colon cancer: findings from the Adjuvant Colon Cancer Endpoints data set
Abstract
Purpose: Limited data exist regarding the outcomes of adjuvant therapy in younger patients with stage II and III colon cancer. We examined disease-free survival (DFS), overall survival (OS), recurrence-free interval (RFI), and grade 3+ adverse events (AEs) in younger patients in the 33,574 patient Adjuvant Colon Cancer Endpoints Group data set.
Patients and methods: Individual patient data from 24 randomized phase III clinical trials were obtained for survival outcomes, which included 10 clinical trials for AE outcomes. Two age-based cutoff points were used to define younger patients: age younger than 40 years and younger than 50 years. Adjuvant therapy benefit analyses were limited to the nine clinical trials in which the investigational chemotherapeutic arm demonstrated benefit.
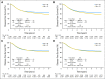
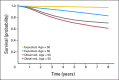
Results: One thousand seven hundred fifty-eight patients (5.2%) were younger than 40 years, 5,817 patients (17.3%) were younger than 50 years, and only 299 patients (0.9%) were younger than 30 years. No meaningful differences in sex or stage were noted in younger versus older patients. Younger and older patients did not differ in RFI (age, < 40 years: hazard ratio [HR], 1.0; P = .62 and age < 50 years: HR, 1.02; P = .35). Younger patients (both cutoff points), had longer OS and DFS than older patients. In trials demonstrating adjuvant therapy benefit, similar DFS benefit was observed by age. Younger patients experienced less leukopenia and stomatitis, but more frequent nausea/vomiting.
Conclusion: Among patients on clinical trials, younger and older patients with stage II and III colon cancer had similar RFI and adjuvant therapy benefit. Younger patients have longer OS and DFS, which is likely primarily because of fewer competing causes of death. Adjuvant therapy is beneficial for colon cancer in patients younger than 50 years who meet typical clinical trial eligibility criteria.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: an analysis of ACCENT adjuvant trials.J Natl Cancer Inst. 2011 Oct 19;103(20):1498-506. doi: 10.1093/jnci/djr310. Epub 2011 Oct 12. J Natl Cancer Inst. 2011. PMID: 21997132 Free PMC article.
-
Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials.Ann Oncol. 2015 Apr;26(4):715-724. doi: 10.1093/annonc/mdv003. Epub 2015 Jan 16. Ann Oncol. 2015. PMID: 25595934 Free PMC article. Clinical Trial.
-
The prognostic impact of primary tumour location in patients with stage II and stage III colon cancer receiving adjuvant therapy. A GISCAD analysis from three large randomised trials.Eur J Cancer. 2019 Apr;111:1-7. doi: 10.1016/j.ejca.2019.01.020. Epub 2019 Feb 21. Eur J Cancer. 2019. PMID: 30797014
-
Adjuvant therapy for colon cancer.Curr Gastroenterol Rep. 2007 Oct;9(5):415-21. doi: 10.1007/s11894-007-0052-x. Curr Gastroenterol Rep. 2007. PMID: 17991344 Review.
-
The role of targeted agents in the adjuvant treatment of colon cancer: a meta-analysis of randomized phase III studies and review.Oncotarget. 2017 May 9;8(19):31112-31118. doi: 10.18632/oncotarget.16091. Oncotarget. 2017. PMID: 28415706 Free PMC article. Review.
Cited by
-
Log odds of positive lymph nodes show better predictive performance on the prognosis of early-onset colorectal cancer.Int J Colorectal Dis. 2023 Jul 11;38(1):192. doi: 10.1007/s00384-023-04490-x. Int J Colorectal Dis. 2023. PMID: 37432563
-
Prevalence of Adverse Event Reporting in Adolescents and Young Adults Enrolled in Cancer Clinical Trials.JCO Oncol Pract. 2023 Nov;19(11):1048-1052. doi: 10.1200/OP.23.00201. Epub 2023 Sep 25. JCO Oncol Pract. 2023. PMID: 37748116 Free PMC article. Review.
-
Evaluation of determinants for age disparities in the survival improvement of colon cancer: results from a cohort of more than 486,000 patients in the United States.Am J Cancer Res. 2020 Oct 1;10(10):3395-3405. eCollection 2020. Am J Cancer Res. 2020. PMID: 33163278 Free PMC article.
-
Colorectal cancer in adolescent and young adults: epidemiology in Japan and narrative review.J Gastrointest Oncol. 2023 Aug 31;14(4):1856-1868. doi: 10.21037/jgo-23-98. Epub 2023 Jul 19. J Gastrointest Oncol. 2023. PMID: 37720434 Free PMC article. Review.
-
Long-term complications in adolescent and young adult leukemia survivors.Hematology Am Soc Hematol Educ Program. 2018 Nov 30;2018(1):146-153. doi: 10.1182/asheducation-2018.1.146. Hematology Am Soc Hematol Educ Program. 2018. PMID: 30504303 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Bleyer A, Choi M, Fuller CD, et al. Relative lack of conditional survival improvement in young adults with cancer. Semin Oncol. 2009;36:460–467. - PubMed
-
- Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: The scope of the problem and criticality of clinical trials. Cancer. 2006;107:1645–1655. - PubMed
-
- Bleyer A. The adolescent and young adult gap in cancer care and outcome. Curr Probl Pediatr Adolesc Health Care. 2005;35:182–217. - PubMed
-
- National Cancer Institute, and the LiveStrong Young Adult Alliance. Bethesda, MD: National Cancer Institute and the Livestrong Young Adult Alliance; 2006. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer Report of the Adolescent and Young Adult Oncology Progress Review Group: Report of the Young Adult Oncology Progress Review Group. NIH Publication No. 06-6067.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

