The rickettsial OmpB β-peptide of Rickettsia conorii is sufficient to facilitate factor H-mediated serum resistance
- PMID: 22615250
- PMCID: PMC3434587
- DOI: 10.1128/IAI.00349-12
The rickettsial OmpB β-peptide of Rickettsia conorii is sufficient to facilitate factor H-mediated serum resistance
Abstract
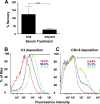
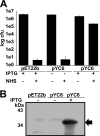
Pathogenic species of the spotted fever group Rickettsia are subjected to repeated exposures to the host complement system through cyclic infections of mammalian and tick hosts. The serum complement machinery is a formidable obstacle for bacteria to overcome if they endeavor to endure this endozoonotic cycle. We have previously demonstrated that that the etiologic agent of Mediterranean spotted fever, Rickettsia conorii, is susceptible to complement-mediated killing only in the presence of specific monoclonal antibodies. We have also shown that in the absence of particular neutralizing antibody, R. conorii is resistant to the effects of serum complement. We therefore hypothesized that the interactions between fluid-phase complement regulators and conserved rickettsial outer membrane-associated proteins are critical to mediate serum resistance. We demonstrate here that R. conorii specifically interacts with the soluble host complement inhibitor, factor H. Depletion of factor H from normal human serum renders R. conorii more susceptible to C3 and membrane attack complex deposition and to complement-mediated killing. We identified the autotransporter protein rickettsial OmpB (rOmpB) as a factor H ligand and further demonstrate that the rOmpB β-peptide is sufficient to mediate resistance to the bactericidal properties of human serum. Taken together, these data reveal an additional function for the highly conserved rickettsial surface cell antigen, rOmpB, and suggest that the ability to evade complement-mediated clearance from the hematogenous circulation is a novel virulence attribute for this class of pathogens.
Figures



Similar articles
-
Pathogenic Rickettsia species acquire vitronectin from human serum to promote resistance to complement-mediated killing.Cell Microbiol. 2014 Jun;16(6):849-61. doi: 10.1111/cmi.12243. Epub 2013 Dec 13. Cell Microbiol. 2014. PMID: 24286496 Free PMC article.
-
Molecular basis of immunity to rickettsial infection conferred through outer membrane protein B.Infect Immun. 2011 Jun;79(6):2303-13. doi: 10.1128/IAI.01324-10. Epub 2011 Mar 28. Infect Immun. 2011. PMID: 21444665 Free PMC article.
-
The Rickettsia conorii Adr1 Interacts with the C-Terminus of Human Vitronectin in a Salt-Sensitive Manner.Front Cell Infect Microbiol. 2017 Mar 1;7:61. doi: 10.3389/fcimb.2017.00061. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28299286 Free PMC article.
-
[Emerging rickettsioses].Parassitologia. 2004 Jun;46(1-2):123-6. Parassitologia. 2004. PMID: 15305700 Review. Italian.
-
Some lessons from Rickettsia genomics.FEMS Microbiol Rev. 2005 Jan;29(1):99-117. doi: 10.1016/j.femsre.2004.09.002. FEMS Microbiol Rev. 2005. PMID: 15652978 Review.
Cited by
-
Pathogenic Rickettsia spp. as emerging models for bacterial biology.J Bacteriol. 2024 Feb 22;206(2):e0040423. doi: 10.1128/jb.00404-23. Epub 2024 Feb 5. J Bacteriol. 2024. PMID: 38315013 Free PMC article. Review.
-
The Retropepsin-Type Protease APRc as a Novel Ig-Binding Protein and Moonlighting Immune Evasion Factor of Rickettsia.mBio. 2021 Dec 21;12(6):e0305921. doi: 10.1128/mBio.03059-21. Epub 2021 Dec 7. mBio. 2021. PMID: 34872352 Free PMC article.
-
Mediterranean Spotted Fever: Current Knowledge and Recent Advances.Trop Med Infect Dis. 2021 Sep 24;6(4):172. doi: 10.3390/tropicalmed6040172. Trop Med Infect Dis. 2021. PMID: 34698275 Free PMC article. Review.
-
Transposon mutagenesis of Rickettsia felis sca1 confers a distinct phenotype during flea infection.PLoS Pathog. 2022 Dec 21;18(12):e1011045. doi: 10.1371/journal.ppat.1011045. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36542675 Free PMC article.
-
Secretome of obligate intracellular Rickettsia.FEMS Microbiol Rev. 2015 Jan;39(1):47-80. doi: 10.1111/1574-6976.12084. Epub 2014 Dec 4. FEMS Microbiol Rev. 2015. PMID: 25168200 Free PMC article. Review.
References
-
- Ahearn JM, Fearon DT. 1989. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21). Adv. Immunol. 46:183–219 - PubMed
-
- Alexander JJ, Hack BK, Cunningham PN, Quigg RJ. 2001. A protein with characteristics of factor H is present on rodent platelets and functions as the immune adherence receptor. J. Biol. Chem. 276:32129–32135 - PubMed
-
- Aslam M, Perkins SJ. 2001. Folded-back solution structure of monomeric factor H of human complement by synchrotron X-ray and neutron scattering, analytical ultracentrifugation, and constrained molecular modeling. J. Mol. Biol. 309:1117–1138 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

