Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells
- PMID: 22615367
- PMCID: PMC3384143
- DOI: 10.1073/pnas.1118979109
Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells
Abstract
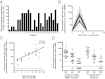
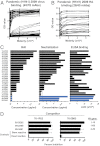
We have previously shown that broadly neutralizing antibodies reactive to the conserved stem region of the influenza virus hemagglutinin (HA) were generated in people infected with the 2009 pandemic H1N1 strain. Such antibodies are rarely seen in humans following infection or vaccination with seasonal influenza virus strains. However, the important question remained whether the inactivated 2009 pandemic H1N1 vaccine, like the infection, could also induce these broadly neutralizing antibodies. To address this question, we analyzed B-cell responses in 24 healthy adults immunized with the pandemic vaccine in 2009. In all cases, we found a rapid, predominantly IgG-producing vaccine-specific plasmablast response. Strikingly, the majority (25 of 28) of HA-specific monoclonal antibodies generated from the vaccine-specific plasmablasts neutralized more than one influenza strain and exhibited high levels of somatic hypermutation, suggesting they were derived from recall of B-cell memory. Indeed, memory B cells that recognized the 2009 pandemic H1N1 HA were detectable before vaccination not only in this cohort but also in samples obtained before the emergence of the pandemic strain. Three antibodies demonstrated extremely broad cross-reactivity and were found to bind the HA stem. Furthermore, one stem-reactive antibody recognized not only H1 and H5, but also H3 influenza viruses. This exceptional cross-reactivity indicates that antibodies capable of neutralizing most influenza subtypes might indeed be elicited by vaccination. The challenge now is to improve upon this result and design influenza vaccines that can elicit these broadly cross-reactive antibodies at sufficiently high levels to provide heterosubtypic protection.
Conflict of interest statement
Conflict of interest statement: R.A., J.W., and P.C.W. have a licensing agreement with MedImmune on the influenza-virus-specific human monoclonal antibodies.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- HHSN266200700006C/AI/NIAID NIH HHS/United States
- G1000758/MRC_/Medical Research Council/United Kingdom
- MC_G1001212/MRC_/Medical Research Council/United Kingdom
- 5U19AI062629-05/AI/NIAID NIH HHS/United States
- U19 AI062629/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- U19 AI057266-06S2/AI/NIAID NIH HHS/United States
- U19-AI057266/AI/NIAID NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- G0902266/MRC_/Medical Research Council/United Kingdom
- U19 AI057266/AI/NIAID NIH HHS/United States
- HHSN266200500026C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

