Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC
- PMID: 22615405
- PMCID: PMC3384197
- DOI: 10.1073/pnas.1203244109
Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC
Abstract
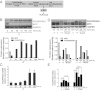
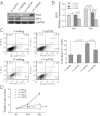
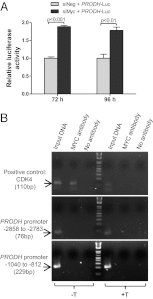
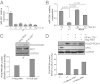
In addition to glycolysis, the oncogenic transcription factor c-MYC (MYC) stimulates glutamine catabolism to fuel growth and proliferation of cancer cells through up-regulating glutaminase (GLS). Glutamine is converted to glutamate by GLS, entering the tricarboxylic acid cycle as an important energy source. Less well-recognized, glutamate can also be converted to proline through Δ(1)-pyrroline-5-carboxylate (P5C) and vice versa. This study suggests that some MYC-induced cellular effects are due to MYC regulation of proline metabolism. Proline oxidase, also known as proline dehydrogenase (POX/PRODH), the first enzyme in proline catabolism, is a mitochondrial tumor suppressor that inhibits proliferation and induces apoptosis. MiR-23b* mediates POX/PRODH down-regulation in human kidney tumors. MiR-23b* is processed from the same transcript as miR-23b; the latter inhibits the translation of GLS. Using MYC-inducible human Burkitt lymphoma model P493 and PC3 human prostate cancer cells, we showed that MYC suppressed POX/PRODH expression primarily through up-regulating miR-23b*. The growth inhibition in the absence of MYC was partially reversed by POX/PRODH knockdown, indicating the importance of suppression of POX/PRODH in MYC-mediated cellular effects. Interestingly, MYC not only inhibited POX/PRODH, but also markedly increased the enzymes of proline biosynthesis from glutamine, including P5C synthase and P5C reductase 1. MYC-induced proline biosynthesis from glutamine was directly confirmed using (13)C,(15)N-glutamine as a tracer. The metabolic link between glutamine and proline afforded by MYC emphasizes the complexity of tumor metabolism. Further studies of the relationship between glutamine and proline metabolism should provide a deeper understanding of tumor metabolism while enabling the development of novel therapeutic strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

