A-type K+ channels encoded by Kv4.2, Kv4.3 and Kv1.4 differentially regulate intrinsic excitability of cortical pyramidal neurons
- PMID: 22615428
- PMCID: PMC3476638
- DOI: 10.1113/jphysiol.2012.229013
A-type K+ channels encoded by Kv4.2, Kv4.3 and Kv1.4 differentially regulate intrinsic excitability of cortical pyramidal neurons
Abstract
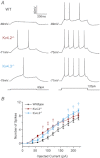
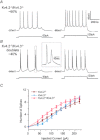
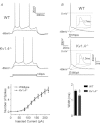
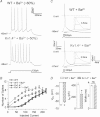
Rapidly activating and rapidly inactivating voltage-gated A-type K+ currents, IA, are key determinants of neuronal excitability and several studies suggest a critical role for the Kv4.2 pore-forming α subunit in the generation of IA channels in hippocampal and cortical pyramidal neurons. The experiments here demonstrate that Kv4.2, Kv4.3 and Kv1.4 all contribute to the generation of IA channels in mature cortical pyramidal (CP) neurons and that Kv4.2-, Kv4.3- and Kv1.4-encoded IA channels play distinct roles in regulating the intrinsic excitability and the firing properties of mature CP neurons. In vivo loss of Kv4.2, for example, alters the input resistances, current thresholds for action potential generation and action potential repolarization of mature CP neurons. Elimination of Kv4.3 also prolongs action potential duration, whereas the input resistances and the current thresholds for action potential generation in Kv4.3−/− and WT CP neurons are indistinguishable. In addition, although increased repetitive firing was observed in both Kv4.2−/− and Kv4.3−/− CP neurons, the increases in Kv4.2−/− CP neurons were observed in response to small, but not large, amplitude depolarizing current injections, whereas firing rates were higher in Kv4.3−/− CP neurons only with large amplitude current injections. In vivo loss of Kv1.4, in contrast, had minimal effects on the intrinsic excitability and the firing properties of mature CP neurons. Comparison of the effects of pharmacological blockade of Kv4-encoded currents in Kv1.4−/− and WT CP neurons, however, revealed that Kv1.4-encoded IA channels do contribute to controlling resting membrane potentials, the regulation of current thresholds for action potential generation and repetitive firing rates in mature CP neurons.
Figures
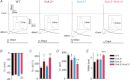


 values in Kv4.2−/−/Kv4.3−/− cells were significantly (
values in Kv4.2−/−/Kv4.3−/− cells were significantly ( P < 0.001;
P < 0.001;  P < 0.01;
P < 0.01;  P < 0.05) different from those in Kv4.3−/− cells, and #values in Kv4.2−/−/Kv4.3−/− cells were significantly (#P < 0.001) different from Kv4.2−/− cells.
P < 0.05) different from those in Kv4.3−/− cells, and #values in Kv4.2−/−/Kv4.3−/− cells were significantly (#P < 0.001) different from Kv4.2−/− cells.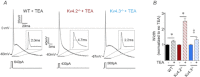
Comment in
-
What does type A mean to a pyramidal cell?J Physiol. 2012 Aug 15;590(16):3643-4. doi: 10.1113/jphysiol.2012.238030. J Physiol. 2012. PMID: 22904364 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

