Roles of individual prolyl-4-hydroxylase isoforms in the first 24 hours following transient focal cerebral ischaemia: insights from genetically modified mice
- PMID: 22615432
- PMCID: PMC3476649
- DOI: 10.1113/jphysiol.2012.232884
Roles of individual prolyl-4-hydroxylase isoforms in the first 24 hours following transient focal cerebral ischaemia: insights from genetically modified mice
Abstract
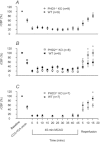
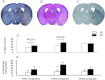
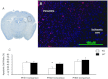
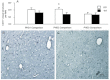
This study investigated the function of each of the hypoxia inducible factor (HIF) prolyl-4-hydroxylase enzymes (PHD1–3) in the first 24 h following transient focal cerebral ischaemia by using mice with each isoform genetically suppressed. Male, 8- to 12-week old PHD1−/−, PHD2+/− and PHD3−/− mice and their wild-type (WT) littermate were subjected to 45 min of middle cerebral artery occlusion (MCAO). During the experiments, regional cerebral blood flow (rCBF) was recorded by laser Doppler flowmetry. Behaviour was assessed at both 2 h and 24 h after reperfusion with a common neuroscore. Infarct volumes, blood–brain barrier (BBB) disruption, cerebral vascular density, apoptosis, reactive oxygen species (ROS), HIF1α, and glycogen levels were then determined using histological and immunohistochemical techniques. When compared to their WT littermates, PHD2+/− mice had significantly increased cerebral microvascular density and more effective restoration of CBF upon reperfusion. PHD2+/− mice showed significantly better functional outcomes and higher activity rates at both 2 h and 24 h after MCAO, associated with significant fewer apoptotic cells in the penumbra and less BBB disruption; PHD3−/− mice had impaired rCBF upon early reperfusion but comparable functional outcomes; PHD1−/− mice did not show any significant changes following the MCAO. Production of ROS, HIF1α staining and glycogen content in the brain were not different in any comparison. Life-long genetic inhibition of PHD enzymes produces different effects on outcome in the first 24 h after transient cerebral ischaemia. These need to be considered in optimizing therapeutic effects of PHD inhibitors, particularly when isoform specific inhibitors become available.
Figures


Similar articles
-
Neuron-specific prolyl-4-hydroxylase domain 2 knockout reduces brain injury after transient cerebral ischemia.Stroke. 2012 Oct;43(10):2748-56. doi: 10.1161/STROKEAHA.112.669598. Epub 2012 Aug 28. Stroke. 2012. PMID: 22933585
-
Morg1(+/-) heterozygous mice are protected from experimentally induced focal cerebral ischemia.Brain Res. 2012 Oct 30;1482:22-31. doi: 10.1016/j.brainres.2012.09.017. Epub 2012 Sep 13. Brain Res. 2012. PMID: 22982595
-
HIF prolyl hydroxylase inhibition prior to transient focal cerebral ischaemia is neuroprotective in mice.J Neurochem. 2014 Oct;131(2):177-89. doi: 10.1111/jnc.12804. Epub 2014 Jul 18. J Neurochem. 2014. PMID: 24974727
-
The regulation, localization, and functions of oxygen-sensing prolyl hydroxylase PHD3.Biol Chem. 2013 Apr;394(4):449-57. doi: 10.1515/hsz-2012-0330. Biol Chem. 2013. PMID: 23380539 Review.
-
HIF-prolyl hydroxylases and cardiovascular diseases.Toxicol Mech Methods. 2012 Jun;22(5):347-58. doi: 10.3109/15376516.2012.673088. Toxicol Mech Methods. 2012. PMID: 22424133 Review.
Cited by
-
FKBP5 Exacerbates Impairments in Cerebral Ischemic Stroke by Inducing Autophagy via the AKT/FOXO3 Pathway.Front Cell Neurosci. 2020 Jul 15;14:193. doi: 10.3389/fncel.2020.00193. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32760250 Free PMC article.
-
Neuroprotection for stroke: current status and future perspectives.Int J Mol Sci. 2012;13(9):11753-11772. doi: 10.3390/ijms130911753. Epub 2012 Sep 18. Int J Mol Sci. 2012. PMID: 23109881 Free PMC article. Review.
-
HIF1α stabilization in hypoxia is not oxidant-initiated.Elife. 2021 Oct 1;10:e72873. doi: 10.7554/eLife.72873. Elife. 2021. PMID: 34596045 Free PMC article.
-
The PHD1 oxygen sensor in health and disease.J Physiol. 2018 Sep;596(17):3899-3913. doi: 10.1113/JP275327. Epub 2018 Mar 5. J Physiol. 2018. PMID: 29435987 Free PMC article. Review.
-
Therapeutic targeting of oxygen-sensing prolyl hydroxylases abrogates ATF4-dependent neuronal death and improves outcomes after brain hemorrhage in several rodent models.Sci Transl Med. 2016 Mar 2;8(328):328ra29. doi: 10.1126/scitranslmed.aac6008. Sci Transl Med. 2016. PMID: 26936506 Free PMC article.
References
-
- Adams HP, Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, Grubb RL, Higashida R, Kidwell C, Kwiatkowski TG, Marler JR, Hademenos GJ. Guidelines for the Early Management of Patients With Ischemic Stroke: A Scientific Statement From the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–1083. - PubMed
-
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the Prolyl Hydroxylases PHD1, PHD2 and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. - PubMed
-
- Aragonés J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. - PubMed
-
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

