Plasticity of NMDA receptor-mediated excitatory postsynaptic currents at perforant path inputs to dendrite-targeting interneurons
- PMID: 22615437
- PMCID: PMC3476632
- DOI: 10.1113/jphysiol.2012.234740
Plasticity of NMDA receptor-mediated excitatory postsynaptic currents at perforant path inputs to dendrite-targeting interneurons
Abstract
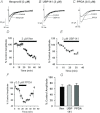
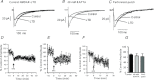
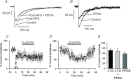
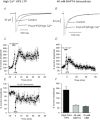
Synaptic plasticity of NMDA receptors (NMDARs) has been recently described in a number of brain regions and we have previously characterised LTP and LTD of glutamatergic NMDA receptor-mediated EPSCs (NMDAR-EPSCs) in granule cells of dentate gyrus. The functional significance of NMDAR plasticity at perforant path synapses on hippocampal network activity depends on whether this is a common feature of perforant path synapses on all postsynaptic target cells or if this plasticity occurs only at synapses on principal cells. We recorded NMDAR-EPSCs at medial perforant path synapses on interneurons in dentate gyrus which had significantly slower decay kinetics compared to those recorded in granule cells. NMDAR pharmacology in interneurons was consistent with expression of both GluN2B- and GluN2D-containing receptors. In contrast to previously described high frequency stimulation-induced bidirectional plasticity of NMDAR-EPSCs in granule cells, only LTD of NMDAR-EPSCs was induced in interneurons in our standard experimental conditions. In interneurons, LTD of NMDAR-EPSCs was associated with a loss of sensitivity to a GluN2D-selective antagonist and was inhibited by the actin stabilising agent, jasplakinolide. While LTP of NMDAR-EPSCs can be readily induced in granule cells, this form of plasticity was only observed in interneurons when extracellular calcium was increased above physiological concentrations during HFS or when PKC was directly activated by phorbol ester, suggesting that opposing forms of plasticity at inputs to interneurons and principal cells may act to regulate granule cell dendritic integration and processing.
Figures





Similar articles
-
Spontaneous and synaptic input from granule cells and the perforant path to dentate basket cells in the rat hippocampus.Hippocampus. 1995;5(3):151-64. doi: 10.1002/hipo.450050302. Hippocampus. 1995. PMID: 7550611
-
Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering.J Neurosci. 2006 Jan 25;26(4):1128-32. doi: 10.1523/JNEUROSCI.2753-05.2006. J Neurosci. 2006. PMID: 16436598 Free PMC article.
-
Synaptic strength at the temporoammonic input to the hippocampal CA1 region in vivo is regulated by NMDA receptors, metabotropic glutamate receptors and voltage-gated calcium channels.Neuroscience. 2015 Nov 19;309:191-9. doi: 10.1016/j.neuroscience.2015.03.014. Epub 2015 Mar 17. Neuroscience. 2015. PMID: 25791230
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
Long-term synaptic plasticity in hippocampal interneurons.Nat Rev Neurosci. 2007 Sep;8(9):687-99. doi: 10.1038/nrn2207. Nat Rev Neurosci. 2007. PMID: 17704811 Review.
Cited by
-
Unlearning: NMDA receptor-mediated metaplasticity in the anterior piriform cortex following early odor preference training in rats.J Neurosci. 2014 Apr 9;34(15):5143-51. doi: 10.1523/JNEUROSCI.0128-14.2014. J Neurosci. 2014. PMID: 24719094 Free PMC article.
-
GluN2B and GluN2D NMDARs dominate synaptic responses in the adult spinal cord.Sci Rep. 2014 Feb 13;4:4094. doi: 10.1038/srep04094. Sci Rep. 2014. PMID: 24522697 Free PMC article.
-
Allosteric modulation of GluN2C/GluN2D-containing NMDA receptors bidirectionally modulates dopamine release: implication for Parkinson's disease.Br J Pharmacol. 2014 Aug;171(16):3938-45. doi: 10.1111/bph.12758. Br J Pharmacol. 2014. PMID: 24818560 Free PMC article.
-
GluN2D-containing NMDA receptors-mediate synaptic currents in hippocampal interneurons and pyramidal cells in juvenile mice.Front Cell Neurosci. 2015 Mar 25;9:95. doi: 10.3389/fncel.2015.00095. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25859181 Free PMC article.
-
NMDA Receptors Containing the GluN2D Subunit Control Neuronal Function in the Subthalamic Nucleus.J Neurosci. 2015 Dec 2;35(48):15971-83. doi: 10.1523/JNEUROSCI.1702-15.2015. J Neurosci. 2015. PMID: 26631477 Free PMC article.
References
-
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. - PubMed
-
- Berretta N, Berton F, Bianchi R, Brunelli M, Capogna M, Francesconi W. Long-term potentiation of NMDA receptor-mediated EPSPs in guinea-pig hippocampal slices. Eur J Neurosci. 1991;3:850–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

