Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice
- PMID: 22615456
- PMCID: PMC3595980
- DOI: 10.1136/annrheumdis-2011-201087
Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice
Abstract
Objective: To evaluate the safety and efficacy of tocilizumab in clinical practice in patients with rheumatoid arthritis (RA) with inadequate responses (IR) to disease-modifying antirheumatic drugs (DMARDs) or both DMARDs and tumour necrosis factor α inhibitors (TNFis).
Methods: Patients-categorised as TNFi-naive, TNFi-previous (washout) or TNFi-recent (no washout) -received open-label tocilizumab (8 mg/kg) every 4 weeks ± DMARDs for 24 weeks. Adverse events (AEs) and treatment discontinuations were monitored. Efficacy end points included American College of Rheumatology (ACR) responses, 28-joint disease activity score (DAS28) and European League Against Rheumatism responses.
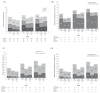
Results: Overall, 1681 (976 TNF-naive, 298 TNFi-previous and 407 TNFi-recent) patients were treated; 5.1% discontinued treatment because of AEs. The AE rate was numerically higher in TNFi-recent (652.6/100 patient-years (PY)) and TNFi-previous (653.6/100PY) than in TNFi-naive (551.1/100PY) patients. Serious AE rates were 18.0/100PY, 28.0/100PY and 18.6/100PY; serious infection rates were 6.0/100PY, 6.8/100PY and 4.2/100PY, respectively. At week 4, 36.5% of patients achieved ACR20 response and 14.9% DAS28 remission (<2.6); at week 24, 66.9%, 46.6%, 26.4% and 56.8% achieved ACR20/ACR50/ACR70 responses and DAS28 remission, respectively. Overall, 61.6% (TNFi-naive), 48.5% (TNFi-previous) and 50.4% (TNFi-recent) patients achieved DAS28 remission.
Conclusions: In patients with RA who were DMARD-IR/TNFi-IR, tocilizumab ± DMARDs provided rapid and sustained efficacy without unexpected safety concerns.
Conflict of interest statement
Figures
References
-
- Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586–93 - PubMed
-
- Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552–63 - PubMed
-
- Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97 - PubMed
-
- Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968–80 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

